Dysphagia is relatively common in individuals with neurological disorders.
ObjectiveTo describe the swallowing management and investigate associated factors with swallowing in a case series of patients with Parkinson's disease.
MethodsIt is a long-term study with 24 patients. The patients were observed in a five-year period (2006–2011). They underwent Fiberoptic Endoscopic Evaluation of Swallowing, Functional Oral Intake Scale and therapeutic intervention every three months. In the therapeutic intervention they received orientation about exercises to improve swallowing. The Chi-square, Kruskal–Wallis and Fisher's tests were used. The period of time for improvement or worsening of swallowing was described by Kaplan–Meier analysis.
ResultsDuring the follow-up, ten patients improved, five stayed the same and nine worsened their swallowing functionality. The median time for improvement was ten months. Prior to the worsening there was a median time of 33 months of follow-up. There was no associated factor with improvement or worsening of swallowing. The maneuvers frequently indicated in therapeutic intervention were: chin-tuck, bolus consistency, bolus effect, strengthening-tongue, multiple swallows and vocal exercises.
ConclusionThe swallowing management was characterized by swallowing assessment every three months with indication of compensatory and rehabilitation maneuvers, aiming to maintain the oral feeding without risks. There was no associated factor with swallowing functionality in this case series.
A disfagia é frequente em indivíduos com distúrbios neurológicos.
ObjetivoDescrever o tratamento da disfagia e investigar fatores associados à deglutição em uma série de casos com doença de Parkinson.
MétodoTrata-se de um estudo longitudinal com 24 pacientes acompanhados por um período de cinco anos (2006–2011). Todos foram submetidos à videoendoscopia da deglutição, classificação de acordo com a Functional Oral Intake Scale (FOIS) e receberam orientações sobre o tratamento da deglutição a cada três meses. As orientações do tratamento da deglutição compreenderam exercícios para a melhora da deglutição. Os testes Qui-quadrado, Kruskal–Wallis e Fisher foram utilizados para investigar associação entre o estado da deglutição e variáveis clínicas.
ResultadosDurante o acompanhamento, dez pacientes melhoraram, cinco mantiveram e nove pioraram a funcionalidade da deglutição. Uma mediana de dez meses foi observada até a melhora na deglutição ser obtida. Foi observada uma mediana de 33 meses de acompanhamento até a piora na deglutição. As manobras mais frequentemente indicadas na terapia foram: queixo para baixo, mudança na consistência e no efeito do bolo, exercícios para força e mobilidade de língua, deglutições múltiplas e exercícios vocais.
ConclusãoO tratamento da disfagia foi caracterizado por avaliações trimestrais da deglutição com indicação de manobras compensatórias e reabilitadoras. Nesta casuística não foram identificados fatores associados às mudanças na funcionalidade da deglutição.
Dysphagia is common in individuals with neurological disorders. It affects food intake, which may lead to complications such as choking, malnutrition, and pulmonary aspiration.1
Parkinson's disease (PD) is one of the most common neurodegenerative disease in the elderly population, with a worldwide incidence between 1 and 20 per 1000people/year.2,3 It is characterized by impairment of basal ganglia in voluntary movements, causing resting tremor, rigidity, akinesia (or bradykinesia), and postural instability.2,4
Dysphagia is very common in PD, affecting over 80% of individuals, reflecting the underlying motor impairments and the extent of the disease's progression.5 The swallowing difficulties most frequently associated with PD are related to the oral and pharyngeal phase, resulting in abnormal bolus formation, delayed swallowing reflex, and prolongation of the pharyngeal transit time, with repetitive swallows to clear the throat.6
These dysphagia-related impairments have a direct influence on the nutritional and health status of the patients, and are associated with increased morbidity and mortality.7,8 However, few studies have described the progression of dysphagia and its severity in PD.9 There is very little information regarding the temporal aspect of dysphagia progression in PD.
Knowledge on dysphagia progression in PD could decrease the risk of aspiration pneumonia, consequently decreasing the risk of death, since it is one of the most frequent causes of death in this patients.10,11 It can also orient physicians and therapists on what to expect of their patients and which treatment may be necessary over time.
Swallowing management, through the utilization of methods that compensate for the alterations in the swallowing process, aims to preserve a safe oral feeding as long as possible. Swallowing management is based on maneuvers that, according to Crary,12 can be categorized as compensatory and rehabilitation maneuvers. Compensatory maneuvers refer to behavioral intervention in dysphagia, characterized by dietary modifications, changes in the manner of administration of the diet, changes in the patient position, and alterations in the mechanism of swallowing. These maneuvers, such as chin-tuck, head rotation, head tilt, head back, among others, are commonly known as postural maneuvers. Their purpose is to direct the bolus and modify the flow velocity of the bolus. The maneuvers characterized by diet modifications promote changes in sensory stimuli, as modifying volume and consistency of the food may alter sensory input. The modification of the swallowing mechanism requires changes in swallowing pattern, regarding muscle strength, range of motion, and coordination of events in swallowing, for example effort swallowing, supraglottic maneuver, multiple swallows, between others.
Among rehabilitation maneuvers are the sensor-motor oral exercises, which enable modifications of force, length, and range of motion of the structures involved in the oral cavity, pharynx, and larynx. Among the most used maneuvers are the shaker maneuver, lingual control, vocal exercises, and pharyngeal exercises.
This article aimed to describe the swallowing management and investigated associated factors with swallowing functionality in a case series of patients with PD.
MethodsSelection of patientsThis was a prospective long-term study of 24 dysphagic patients with idiopathic PD, followed-up for a five-year period (2006–2011) in a large Brazilian university hospital. This was an open case series and, during the five years of study, some patients dropped out. The patients were in use of medicines with Levodopa.
Only the patients who had at least three evaluations, complaints of swallowing, exclusive oral feeding, and regular check-ups with an outpatient neurologist were included in the study. Patients with non-oral feeding and concomitant diseases or disorders that could cause dysphagia were excluded.
ProceduresPatients underwent swallowing evaluation, assessment through the Functional Oral Intake Scale (FOIS),13 and therapeutic intervention every three months.
The swallowing evaluation was obtained through a fiberoptic endoscopic evaluation of swallowing (FEES) and clinically. In both evaluations, three types of food were offered: (1) lemon juice colored with green food coloring; (2) Nectar, honey, and pudding consistencies, all colored with green food coloring (these fluids were obtained with the addition of two, three, and four teaspoons of a thickener [Thicken-easy®] to 100mL of water, respectively, and were offered in two different quantities, 3mL and 7mL); (3) solid consistency, represented by a cornstarch biscuit.
The food was given to patients in the following sequence: liquid and nectar (3mL and 3mL, respectively, followed by 7mL and 7mL, respectively); honey (3mL and 3mL, respectively, followed by 7mL and 7mL, respectively); pudding (two tablespoons); solid (½ cornstarch biscuit). The liquid food was administered in 20mL syringes, and the sample was introduced into the patient's oral cavity. As difficulties in swallowing were observed, airway protective maneuvers and/or changes in head posture were performed in order to assist oral feeding in a safe way.
FEES was conducted by an otolaryngologist, while the food was offered by a speech-language therapist.
In the clinical evaluation, as the patients swallowed, cervical auscultation was performed to identify abnormal signs at the pharyngeal swallowing phase. Oral bolus transit time, anterior or posterior escape, positive cervical auscultation (with signs that indicate a presence of stasis or penetration with aspiration risk), coughing (before, during, or after swallowing), and wet voice were also observed by a speech-language therapist.
Based on the clinical evaluation and FEES, the FOIS13 was applied. The FOIS ranks patients into levels, adapted to the present study as follows: level 1, No oral feeding; Level 2, tube-dependent with minimal attempts of food or liquid; Level 3, Tube-dependent with consistent oral intake of food or liquid; Level 4, Complete oral diet of one or two consistencies (nectar and honey, honey and pudding); Level 5a, Complete oral diet with multiple consistencies but with restrictions in two consistencies (for example, solid and liquid), with or without compensation; Level 5b, Complete oral diet with multiple consistencies but with restriction of one consistency (for example, solid or liquid), with or without compensation; Level 5c, Complete oral diet with multiple consistencies, but requiring compensation; Level 6, Complete oral diet with multiple consistencies without special preparation but with specific food limitations (for example: fibers, grains, and some vegetables) and speed and volume modification if necessary; and Level 7, Complete oral diet with no restriction.
At the initial evaluation, every patient received a corresponding FOIS classification. Every time the patients returned to the hospital they were reevaluated and reclassified under FOIS. To analyze the swallowing functionality by Kaplan–Meier survival analyses, patients were observed until occurrence of the event improvement or the event worsening, i.e., they were observed until the point of the time at which a fall or rise in FOIS was noted. Patients whose swallowing functionality stayed the same were observed during the follow-up period and no event was registered.
Following these swallowing evaluations, patients received therapeutic intervention every three months regarding adequate food consistency and volume, in addition to maneuvers or exercises to improve swallowing functionality. They were oriented to perform the maneuvers daily and received written instructions for each one.
The compensatory maneuvers used were (1) chin-tuck, to improve airway protection during swallowing; (2) bolus consistency, to facilitate the feeding of patients with decreased coordination of tongue, reduced contraction of pharynges, delay in triggering swallowing reflex, reduced airway protection and chewing difficulty; (3) effortful swallow, to increase strength to eject the bolus and to approximate the larynx structures, improving airway protection; (4) frequency of swallowing (multiple swallows), to clear stasis; and (5) bolus effects, i.e. changes in volume, viscosity, temperature, or taste, were introduced in order to improve oral and pharyngeal sensibility and control bolus management.
The following rehabilitation maneuvers were used (1) tongue strengthening, to increase strength to eject the bolus; (2) tongue control, to improve tongue mobility and facilitate the bolus management in the oral cavity; (3) Shaker maneuver, to increase strength in supra hyoid muscles reducing the penetration and aspiration risk due to stasis in pyriform sinus; (4) vocal exercises, to improve airway protection though the improvement in the glottis adduction; (5) tongue holding (Masako maneuver), to increase movements of pharyngeal muscles against the basis of the tongue during the act of swallowing.
Data analysisThe patients were classified according to changes in swallowing functionality measured by FOIS, grouped as: improved, no change, and worsened.
In order to investigate factors related to swallowing functionality, the Chi-square, Kruskal–Wallis, and Fisher's tests were used. The gender and age at first evaluation, age at onset of symptoms, and disease duration (disease duration=time between the onset of PD symptoms and the first evaluation) were considered as independent variables.
The swallowing functionality over time was described by Kaplan–Meier Survival Analysis. It is important to mention that the results of a survival analysis express the probability of the patient not suffering from a given event over time.
The statistical analysis was performed using Statistical Package for the Social Sciences (SPSS), version 13.0 for Windows, and p-values lower than 0.05 were considered significant.
This study was approved by the Ethic Board Committee of the University of Campinas (Protocol number 796/2005).
ResultsThe group of 24 patients comprised 16 men and 8 women. The average age of onset of PD symptoms was 53.8 (±6.5) years. The average age for the first evaluation was 65.4 (±8.6) years. The average disease duration was 139.2 (±65) months, i.e., an average of 11 years between the first symptoms and the first evaluation for swallowing management.
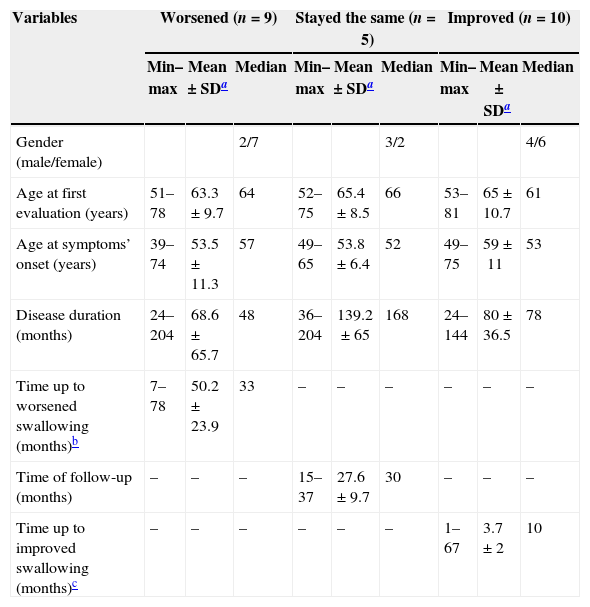
Ten patients improved, five presented no change, and nine worsened their swallowing functionality during follow-up. The characteristics of these groups are described in Table 1. There was no statistically significant difference between the groups.
Descriptive analysis of clinical aspects of a case series of patients with Parkinson's disease followed in a dysphagia outpatient between 2006 and 2011, stratified by swallowing functionality (n=24).
| Variables | Worsened (n=9) | Stayed the same (n=5) | Improved (n=10) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Min–max | Mean±SDa | Median | Min–max | Mean±SDa | Median | Min–max | Mean±SDa | Median | |
| Gender (male/female) | 2/7 | 3/2 | 4/6 | ||||||
| Age at first evaluation (years) | 51–78 | 63.3±9.7 | 64 | 52–75 | 65.4±8.5 | 66 | 53–81 | 65±10.7 | 61 |
| Age at symptoms’ onset (years) | 39–74 | 53.5±11.3 | 57 | 49–65 | 53.8±6.4 | 52 | 49–75 | 59±11 | 53 |
| Disease duration (months) | 24–204 | 68.6±65.7 | 48 | 36–204 | 139.2±65 | 168 | 24–144 | 80±36.5 | 78 |
| Time up to worsened swallowing (months)b | 7–78 | 50.2±23.9 | 33 | – | – | – | – | – | – |
| Time of follow-up (months) | – | – | – | 15–37 | 27.6±9.7 | 30 | – | – | – |
| Time up to improved swallowing (months)c | – | – | – | – | – | – | 1–67 | 3.7±2 | 10 |
None of the investigated variables was statistically associated with swallowing functionality in this case series.
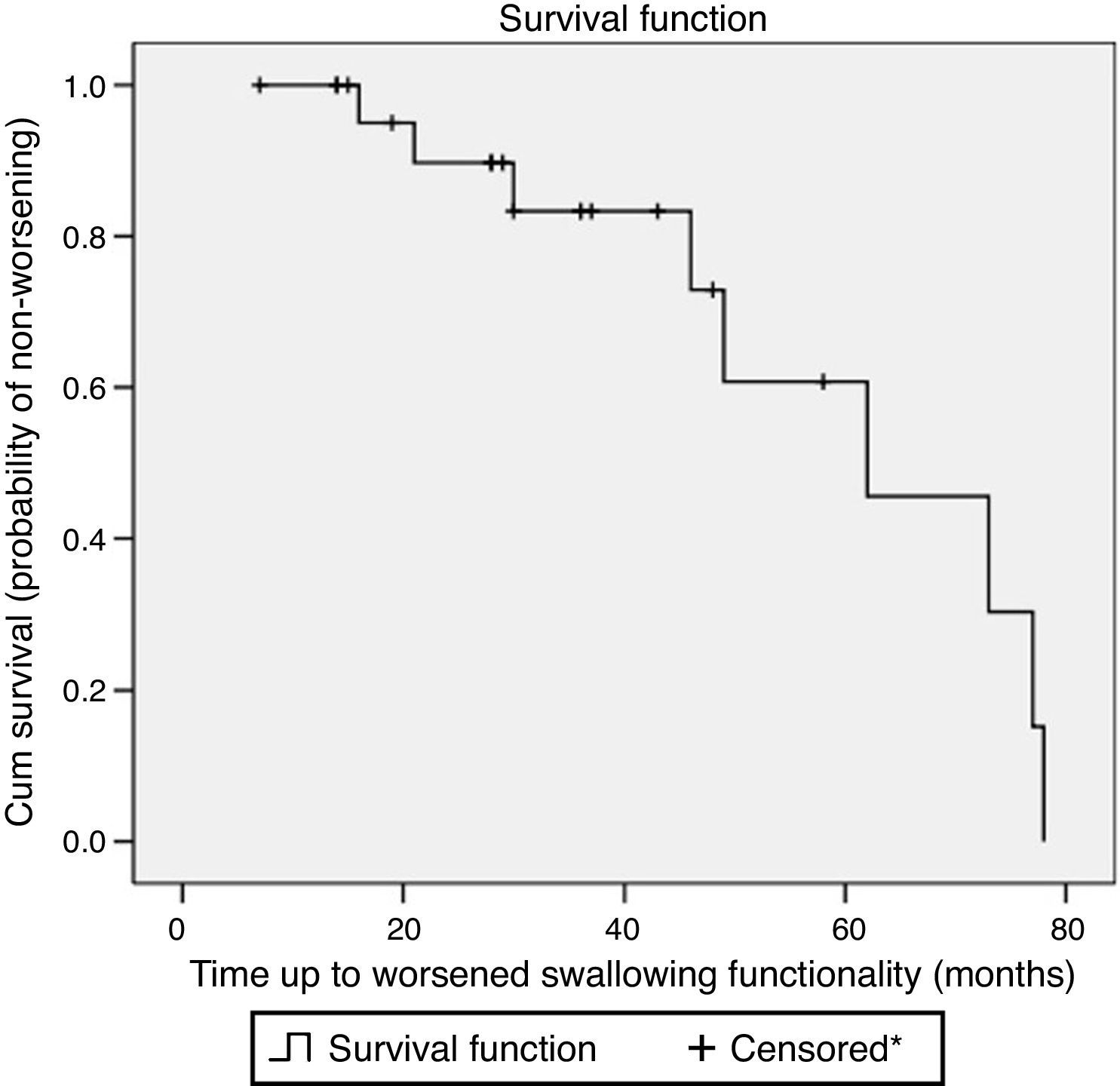
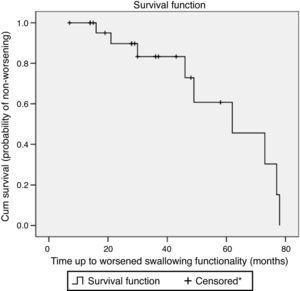
Fig. 1 shows the survival curve of the worsened swallowing group. This curve illustrates the chance of not suffering from worsening of swallowing in this case series.
Survival plot considering worsened swallowing according to Functional Oral Intake Scale (FOIS) levels in Parkinson's disease patients followed at a dysphagia outpatient between 2006 and 2011 (n=24). *Censored observation shows patients lost in follow-up or patients without worsened swallowing functionality during the observation period.
The patients gradually lost swallowing functionality. According to Kaplan–Meier analysis, they had 17% probability of worsened swallowing functionality at the tenth month of follow-up.
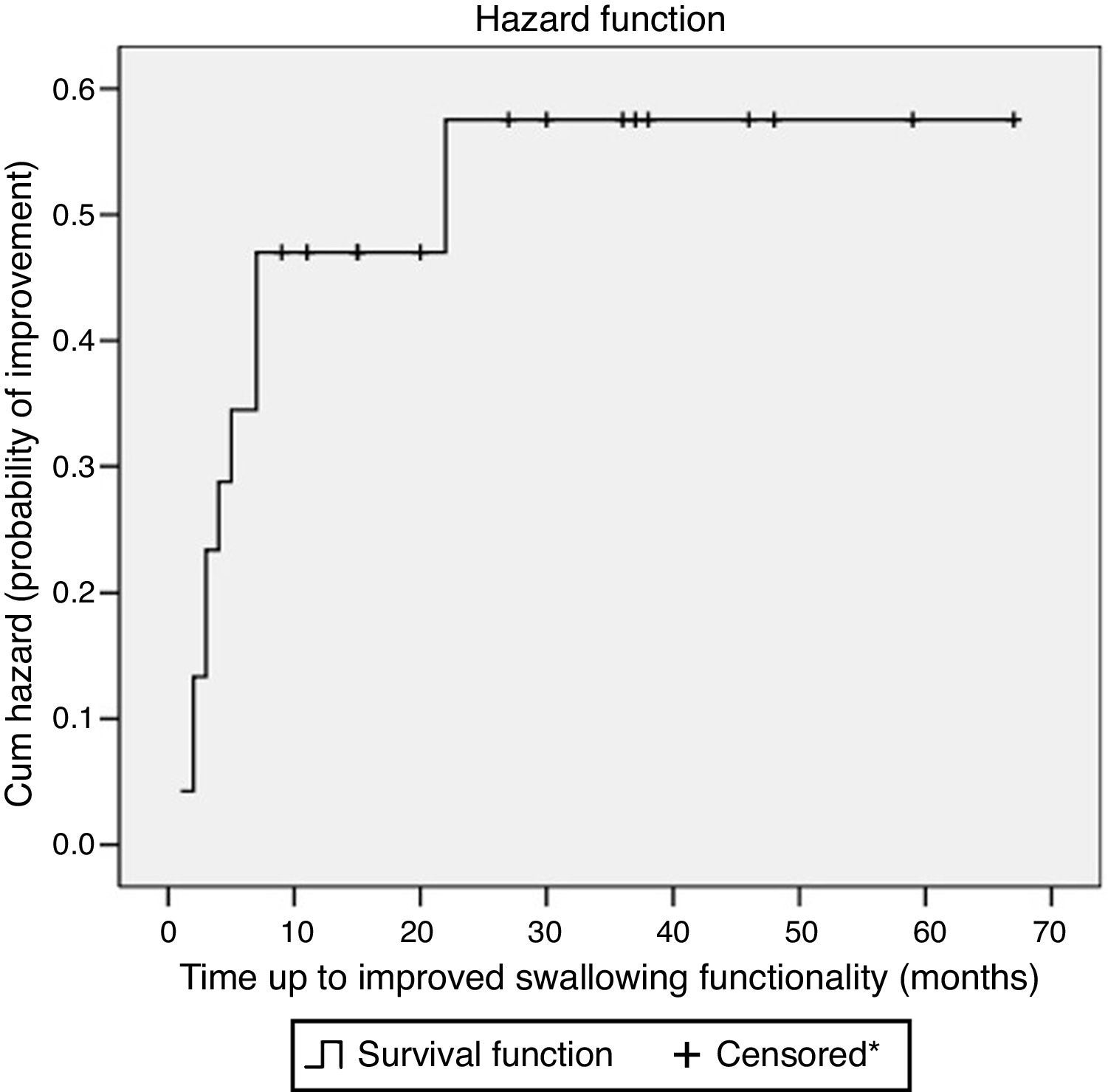
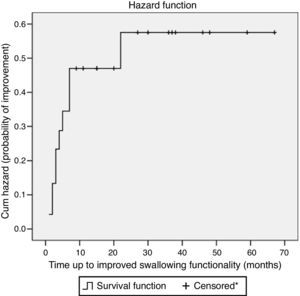
Fig. 2 shows the hazard function of the improvement of swallowing group. This curve illustrates the chance of swallowing improvement in this case series. The majority had improved swallowing functionality up to the tenth month. According to the Kaplan–Meier analysis, in ten months of follow-up, the probability of improvement in swallowing functionality was 44%.
Hazard plot considering the improved swallowing according to Functional Oral Intake Scale (FOIS) levels in Parkinson's disease patients followed at a dysphagia outpatient between 2006 and 2011 (n=24). *Censored observation shows patients lost to follow-up or patients without improved swallowing functionality during the observation period.
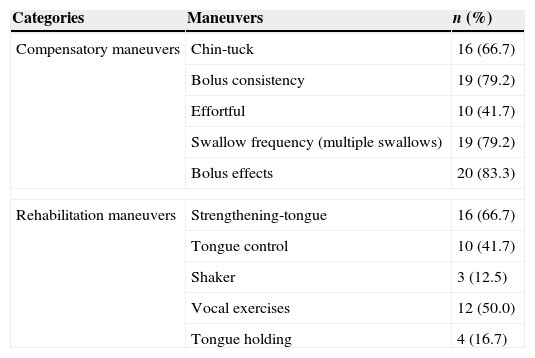
The frequencies of maneuvers indicated in the therapeutic intervention are shown in Table 2. The maneuvers chin-tuck, bolus consistency, tongue strengthening, vocal exercises, swallow frequency, and bolus effect were suggested to 50% or more of the patients.
Frequency of maneuvers recommended in the swallowing management in a case series of patient with Parkinson's disease followed in a dysphagia outpatient between 2006 and 2011 (n=24).
| Categories | Maneuvers | n (%) |
|---|---|---|
| Compensatory maneuvers | Chin-tuck | 16 (66.7) |
| Bolus consistency | 19 (79.2) | |
| Effortful | 10 (41.7) | |
| Swallow frequency (multiple swallows) | 19 (79.2) | |
| Bolus effects | 20 (83.3) | |
| Rehabilitation maneuvers | Strengthening-tongue | 16 (66.7) |
| Tongue control | 10 (41.7) | |
| Shaker | 3 (12.5) | |
| Vocal exercises | 12 (50.0) | |
| Tongue holding | 4 (16.7) | |
In this case series, an average of 11 years between PD onset and the patient's first evaluation at the dysphagia outpatient clinic was observed. This could be explained by several factors, including lack of information or latency between the PD onset and the beginning of swallowing complaints. The literature indicates that the delay in seeking professional assistance may be damaging, especially since dysphagia is prevalent in long-standing PD, but may be subclinical specially in the early course of the disease.5,14 Objective swallowing evaluations have repeatedly found impaired swallowing in over 50% of patients with PD who reported no swallowing abnormalities.14 During the disease course, 75–97% of the patients will suffer from dysphagia.15–17 Because of these evidences, it is important to pay attention to swallowing, always monitoring for weight loss, malnutrition, and pulmonary aspects in order to avoid dysphagia complications.
Müller et al.,18 in a postmortem study, found a median age at PD onset of 60 years, a median survival time of approximately 14 years, and a dysphagia latency of 10 years. The authors demonstrated that disease duration is important, because the latency of dysphagia complaints was positively correlated with the total survival time.
The present study found no association between gender and swallowing functionality in PD. In the literature, a relationship between bronchoaspiration and gender was also not observed.19,20 These facts reveal that aspects related to gender, such as hormones, anatomic differences, among other gender differences, appear to not influence swallowing of PD patients.
Although the progressive impairment of swallowing is an expected fact, in this study, maintenance or improvement of swallowing functionality was observed in the majority of the patients during the follow-up period.
There was no statistic difference found between the groups that presented no change, improved, or worsened swallowing functionality. Although this study did not find aspects statistically associated with swallowing changes in PD patients, Lorefalt et al.21 reported a significant reduction of solid food intake in older patients with PD. The non-ingestion of solid foods per se indicates a loss in FOIS. In PD, higher mortality is associated with some aspects, such as older age, dysphagia, and late diagnosis.22 According to Auyeung et al.23 the older onset is associated with a negative impact on survival of PD patient.
An earlier first evaluation and start of swallowing management in PD patient is very important. Manor et al.8 observed that, in PD, swallowing management in earlier stages of dysphagia was able to prevent aspiration pneumonia and help in the maintenance of the quality of life. Therefore, early swallowing management may positively affect swallowing functionality. According to the literature, compensatory maneuvers used in swallowing management can improve airway protection and reduce dysphagia complications.24
Despite a lack of blind controlled studies and although PD is a progressive and neurodegenerative disease, there are other studies indicating that improvement can be brought by relatively simple interventions.25,26 Although the present study did not aim to verify the efficacy of the methods of therapeutic intervention, the patients in this swallowing management group showed improvement in the first ten months. According to Robbins,12 when some maneuvers are performed repeatedly, they promote neuroplasticity and improve the swallowing functionality.
Swallowing management in PDThe swallowing management of patients with neurodegenerative diseases is relatively recent; for many years, it was believed that degenerative disease would have a consequent progressive dysphagia; swallowing could not be rehabilitated, and thus any attempt to therapeutic intervention would be doomed to failure.
The swallowing management aims to improve the swallowing act as much as possible and to compensate for what cannot be solved, in order to save deglutition.
As a result of this study, it was observed that one of the most recommended interventions were compensatory. The greater use of compensatory maneuvers in PD can be explained by the instantaneous result of these in swallowing, what can be verified empirically in clinical and instrumental evaluation.
Compensatory maneuvers are designed to redirect the bolus away from the airway, without changing airway physiology. The literature elucidates that thickening liquids to a nectar or honey consistency should be used because it exhibits an immediate effect.27 Chin-tuck maneuver is frequently recommended for swallowing thin liquids.28 In PD, there are evidences that the chin-tuck maneuver combined with thick liquid can be important in preventing pneumonia.20
In this swallowing management program, bolus consistency was indicated when other maneuvers were inefficient to maintain oral feeding and it was necessary to avoid the consistencies that were hazardous.
For the patient of this study, the bolus effect maneuver was indicated when the lack of oral control made it difficult to swallow quickly and in greater volumes. The bolus effect, and changes in the consistency, taste, or temperature of the bolus, improve oral intake and prevent aspiration.
Other frequently indicated maneuvers in this swallowing management were frequency or multiple swallowing. They were indicated to be performed during the daily feeding, aiming to clear stasis and reduce the chance of larynx penetration or aspiration.
In rehabilitation maneuvers, the exercises of tongue strengthening and tongue control were frequently indicated because tongue movements of PD patients, especially those responsible for propulsion and chewing, are considered hypokinetic in the oral phase. In these patients the swallowing oral phase is usually longer and even slower than the pharyngeal phase.29
For half of the patients, vocal exercises were indicated, as there are evidences that this type of exercises works to improve the cough function and tongue's mobility during swallowing,27 which leads to a better swallowing functionality and less bronchoaspiration.
Future studies about the efficacy of intervention methods in swallowing of PD patients are necessary. The improvement and maintenance, not expected in neurodegenerative diseases, found in the present study, suggest that swallowing management might positively affect swallowing functionality of PD patients, but it cannot be affirmed due to the design of this study.
In conclusion, the swallowing functionality was characterized by maintenance or improvement, especially in the first ten months of follow-up, comprising compensatory and rehabilitation maneuvers, reoriented each three months. There was no associated factor with changes in swallowing functionality in these cases.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Luchesi KF, Kitamura S, Mourão LF. Dysphagia progression and swallowing management in Parkinson's disease: an observational study. Braz J Otorhinolaryngol. 2015;81:24–30.
Institution: Universidade Estadual de Campinas, Campinas, SP, Brazil.