Several studies have associated congenital sensorineural hearing loss in children with prolongation of the cardiac parameter QTc. The cause of this association is unknown. At the same time, mutations in GJB2, which encodes connexin 26, are the most common cause of congenital hearing impairment.
ObjectiveTo compare electrocardiographic parameters (PR interval, QRS complex, and QTc interval) in patients with hearing loss who were tested for mutations in GJB2 and GJB6 to investigate whether these mutations affect electrical activity of the heart.
Methods346 patients (176 males, 170 females) with sensorineural hearing loss of 30dB HL or more, aged 21.8±19.9 years (including 147 children <14 years), underwent both genetic study for GJB2 and GJB6 mutations and electrocardiography.
ResultsMutations in GJB2, including homozygotes and heterozygotes, were found in 112 (32%) patients. There were no significant differences in ECG parameters between groups of patients with and without mutations in GJB2. No differences were observed either in men (mean PR with mutation: 155±16.6 vs. 153.6±30.1 without; QRS: 99.9±9.9 vs. 101.1±15.4; QTc: 414.9±29.9 vs. 412.4±25.7) or women (mean PR with: 148.7±21 vs. 143.8±22.8 without; QRS: 94.8±7.6 vs. 92.9±9.6; QTc: 416.8±20.6 vs. 424.9±22.8). In similar fashion, we did we find any significant differences between groups of children with and without GJB2 mutations (mean PR with: 126.3±19.6 vs. 127±19.7 without; QRS: 80.7±9.5 vs. 79.4±11.6; QTc: 419.7±23.5 vs. 419.8±24.8).
ConclusionNo association was found between the presence of GJB2 mutations encoding connexin 26 in patients with hearing loss and their ECG parameters (PR, QRS, QTc).
Vários estudos têm associado a perda auditiva neurossensorial congênita em crianças ao prolongamento do parâmetro cardíaco QTc. A causa desta associação é desconhecida. Ao mesmo tempo, as mutações no GJB2, que codifica a conexina 26, são a causa mais comum de deficiência auditiva congênita.
ObjetivoComparar parâmetros eletrocardiográficos (intervalo PR, complexos QRS e intervalo QTc) em pacientes com perda auditiva que foram testados para mutações no GJB2 e GJB6 para investigar se estas mutações afetam a atividade elétrica do coração.
MétodoAo todo, 346 pacientes (176 homens, 170 mulheres) com perda auditiva neurossensorial de 30dB ou mais, com média de idade de 21,8 ± 19,9 anos (incluindo 147 crianças <14 anos), foram submetidos a estudo genético para mutações de GJB2 e GJB6 e eletrocardiograma.
ResultadosMutações no GJB2, incluindo homozigóticos e heterozigóticos, foram encontradas em 112 (32%) pacientes. Não houve diferenças significativas nos parâmetros de ECG entre grupos de pacientes com e sem mutações no GJB2. Não foram observadas diferenças em homens (PR médio com mutação: 155 ± 16,6 vs 153,6 ± 30,1 sem mutação; QRS: 99,9 ± 9,9 vs 101,1 ± 15,4; QTc: 414,9 ± 29,9 vs 412,4 ± 25,7) nem em mulheres (PR médio com: 148,7 ± 21 vs. 143,8 ± 22,8, sem; QRS: 94,8 ± 7,6 vs 92,9 ± 9,6; QTc: 416,8 ± 20,6 vs 424,9 ± 22,8). Da mesma forma, encontramos diferenças significativas entre os grupos de crianças com e sem mutações de GJB2 (PR médio com: 126,3 ± 19,6 vs 127 ± 19,7, sem; QRS: 80,7 ± 9,5 vs 79,4 ± 11,6; QTc: 419,7 ± 23,5 vs. 419,8 ± 24,8).
ConclusãoNão foi encontrada associação entre a presença de mutações de GJB2 que codificam conexina 26 em pacientes com perda auditiva e seus parâmetros de ECG (PR, QRS, QTc).
Connexins are integral transmembrane proteins which form channels in the cell membrane called connexons. In adjacent cells, connexons form tight junctions that are necessary for transfer of electrical signals and water-soluble molecules. Connexons help maintain tissue homeostasis and assist in the management and regulation of a cell's ionic environment.1
Connexins are present throughout the human body. Even though cells can contain a number of different connexins, each plays a separate role. In humans, there are 21 proteins belonging to the connexin family, and each is encoded by a different gene.2 Mutations in genes encoding connexins create disturbances in their structure and function, which can lead to hereditary diseases such as deafness, cataracts, skin diseases, and neurodegenerative conditions.3–6
In the cells of the inner ear, connexin 26 and connexin 30 are involved in potassium ion transport processes necessary for hearing. When a hair cell is stimulated, potassium ions in the endolymph enter the cilia and body of the cell, causing it to depolarize. Due to gap-junction connections, ions are in turn transported by supporting cells and cells in stria vascularis to endolymph. Via this recirculation of potassium ions, a highly positive (+80mV) endolymphatic potential is generated, a necessary condition for hearing. Disruption of this mechanism causes potassium ion intoxication and tissue damage.7
Both the GJB2 (MIM*121011) gene (encoding connexin 26) and the GJB6 (MIM*604418) gene (encoding connexin 30) are located on chromosome 13 (13q11–q12). Mutations of the GJB2 gene lead to disturbances in the functioning of connexin 26, causing hearing loss (HL) by impairing transfer of intracellular potassium ions within the inner ear.8 GJB2 mutations are a major cause of autosomal recessive nonsyndromic HL in many populations. Among Europeans, the most common pathogenic mutation in the GJB2 gene is a deletion of guanine at position 35 (NM_004004.5:c.35delG).9,10 In most cases (70%), HL occurs as an isolated defect; in other instances it is accompanied by heart, craniofacial, kidney, skin, and other defects.
Several studies have reported an association of congenital sensorineural HL with prolongation of QTc interval in children. The cause of this association is unknown.11,12
This study measured electrocardiographic parameters in patients with HL, who were also tested for genetic mutation. Analysis of the electrocardiogram (ECG) provided PR interval, QRS complex, and QTc interval, and these electrocardiographic parameters were compared in two groups of patients: one with HL caused by mutations in the GJB2 gene and another with HL and no mutation in this gene. In this way, it was possible to investigate whether there was a relationship between connexin 26 and connexin 30 functions and cardiac electrical activity.
MethodsThe study populationThe study group consisted of 346 consecutive patients, aged 21.8±19.9 years (0.16–84 years), including 176 males and 170 females, with sensorineural HL of 30dB or more (calculated as the average hearing threshold at frequencies relevant for speech – 0.5, 1, 2, and 4kHz) and on whom genetic tests were done to identify mutations in GJB2 and GJB6. The HL was confirmed by pure tone audiometry or, in the case of children less than 6 years old, Brainstem Evoked Response Audiometry (BERA). Among the subjects studied, 91 had cochlear implants.
Genetic testsDNA was extracted from the peripheral blood by a salting-out procedure.13 The typing for the c.35delG mutation in GJB2 gene was performed by two independent methods: AS-PCR and PCR-RFLP, as described previously.14,15 Briefly, multiplex amplification of three GJB2 regions (c.12-72, c.68-198 and c.306-464) with fluorescent labeled primers was performed. In parallel, PCR-RFLP analysis specific for c.35delG mutation was conducted. Size of the fragments generated by the two above-mentioned protocols was determined using a 3500XL Genetic Analyzer (Life Technologies) with Gene Mapper (Life Technologies) software. Samples lacking two c.35delG mutations were further analyzed by direct DNA sequencing of the PCR amplified exon 2 of the gene. Sequence analysis was performed with Variant Reporter v1.1 (Life Technologies) software. Genotyping of the c.-23+1G>A GJB2 mutation was performed using real-time PCR according to the manufacturer's instructions (Life Technologies), while the GJB6 mutation del (GJB6-D13S1830) was detected using the method of del Castillo et al.16 Genetic nomenclature is in line with recommendations of the Human Genome Variation Society (HGVS).
Electrocardiographic testsAll 346 patients underwent a 12 lead Electrocardiogram (ECG). ECG recording was performed and evaluated using the Sentinel Cardiology Information Management System (Reynolds Medical). The durations of PR interval, QRS complex, and QTc interval were measured by a single observer on a 5 times enlarged image on a computer monitor with a cursor accuracy of 1ms (sampling frequency 1000Hz). PR interval was usually measured in lead II, QRS complex in V1 and/or V5, and QT interval in at least three leads of the same cycle (usually leads II, V2, and V5). Heart rate was calculated from the RR interval preceding the cycle of the QT interval measured. To correct QT interval due to heart rate (i.e., QTc), Bazett's formula was used for rates between 50 and 120bpm; for faster or slower rates Hodges’ formula was used. In order to calculate average QTc, patients with QRS complexes ≥120ms were excluded. A QTc interval above 460ms in women, 450ms in men, and 440ms in children under 14 years of age, was diagnosed as prolonged.
The study population was divided into three groups based on reference values of QTc for children, women, and men,17 giving 147 children <14 years (42.4% of patients), 100 women ≥14 years (28.9%), and 99 men ≥14 years (28.6%). The group of children included 70 (47%) girls.
Statistical analysisStatistical analysis of each of the three groups involved a Kruskal–Wallis test and a Wilcoxon two-sample test.
OtherThe study was approved by the Bioethics Committee, approval number KB/05/2009. The study was done in accordance with the principles of the Declaration of Helsinki. All patients or their guardians gave written consent to participate.
ResultsCharacteristics of the study population and genetic studiesMutations in GJB2 were identified in 112 (32%) patients with HL. No mutations were detected in the GJB2 and GJB6 genes analyzed in the other 234 (68%).
The mean age of children with a GJB2 mutation and children without GJB2 mutation was similar (6.0±4.6 vs. 5.8±4.5 years, p=0.86). The mean age of adults in both groups (with and without GJB2 mutation) was also similar (35.1±17.6 vs. 30.5±16.5 years, p=0.17 for women; 31.6±18.9 and 30.3±17.5 years, p=0.99 for men) (Table 1).
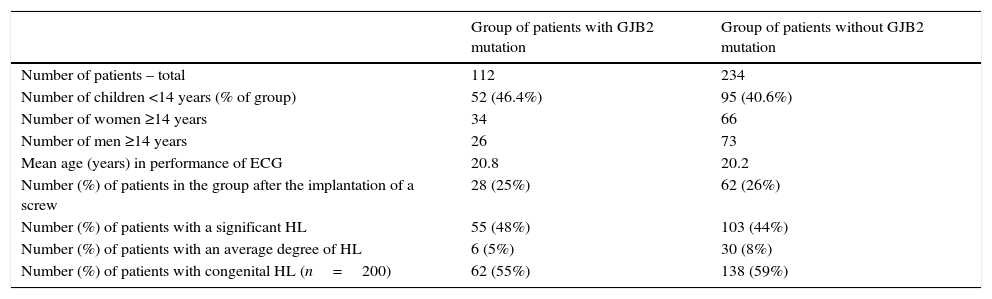
Characteristics of the group of patients diagnosed with mutations in gene GJB2 (genotype homozygous and heterozygous together) and the group of patients without any mutation in the gene.
| Group of patients with GJB2 mutation | Group of patients without GJB2 mutation | |
|---|---|---|
| Number of patients – total | 112 | 234 |
| Number of children <14 years (% of group) | 52 (46.4%) | 95 (40.6%) |
| Number of women ≥14 years | 34 | 66 |
| Number of men ≥14 years | 26 | 73 |
| Mean age (years) in performance of ECG | 20.8 | 20.2 |
| Number (%) of patients in the group after the implantation of a screw | 28 (25%) | 62 (26%) |
| Number (%) of patients with a significant HL | 55 (48%) | 103 (44%) |
| Number (%) of patients with an average degree of HL | 6 (5%) | 30 (8%) |
| Number (%) of patients with congenital HL (n=200) | 62 (55%) | 138 (59%) |
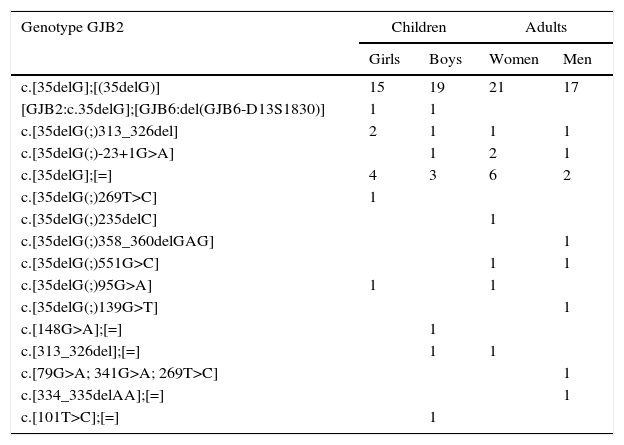
The homozygous genotype c.[35delG];[(35delG)] in the GJB2 gene was detected in 72 (20.8%) subjects. In 40 (11.8%) individuals, heterozygous GJB2 genotypes were identified: c.[35delG];[=] (n=15), c.[35delG(;)313_326del] (n=5), c.[35delG(;)-23+1G>A] (n=4), c.[313_326del];[=] (n=2), c.[35delG(;)551G>C] (n=2), c.[35delG(;)95G>A] (n=2), and a compound GJB2 and GJB6 heterozygous genotype [GJB2:c.35delG];[GJB6:del(GJB6-D13S1830)] (n=2). The following GJB2 heterozygous genotypes occurred in individual cases: c.[35delG(;)269T>C], c.[148G>A];[=], c.[79G>A; 341G>A; 269T>C], c.[101T>C];[=], c.[35delG(;)235delC], c.[35delG(;)358_360delGAG], c.[35delG(;)139G>T], c.[334_335delAA];[=] (Table 2).
Types of genotype and their distribution in the study population.
| Genotype GJB2 | Children | Adults | ||
|---|---|---|---|---|
| Girls | Boys | Women | Men | |
| c.[35delG];[(35delG)] | 15 | 19 | 21 | 17 |
| [GJB2:c.35delG];[GJB6:del(GJB6-D13S1830)] | 1 | 1 | ||
| c.[35delG(;)313_326del] | 2 | 1 | 1 | 1 |
| c.[35delG(;)-23+1G>A] | 1 | 2 | 1 | |
| c.[35delG];[=] | 4 | 3 | 6 | 2 |
| c.[35delG(;)269T>C] | 1 | |||
| c.[35delG(;)235delC] | 1 | |||
| c.[35delG(;)358_360delGAG] | 1 | |||
| c.[35delG(;)551G>C] | 1 | 1 | ||
| c.[35delG(;)95G>A] | 1 | 1 | ||
| c.[35delG(;)139G>T] | 1 | |||
| c.[148G>A];[=] | 1 | |||
| c.[313_326del];[=] | 1 | 1 | ||
| c.[79G>A; 341G>A; 269T>C] | 1 | |||
| c.[334_335delAA];[=] | 1 | |||
| c.[101T>C];[=] | 1 | |||
The next phase of the study was to compare electrocardiographic parameters in the study population. The measured parameters were compared between two groups: the group of patients with a mutation in gene GJB2 (both homozygotes and heterozygotes) and the group of patients with no mutation in the gene. No significant differences were found for any measured parameter in any of the groups studied. Table 3 presents inter-group differences in PR interval; Table 4 presents inter-group differences in QRS complex; and Table 5 presents inter-group differences in QTc interval.
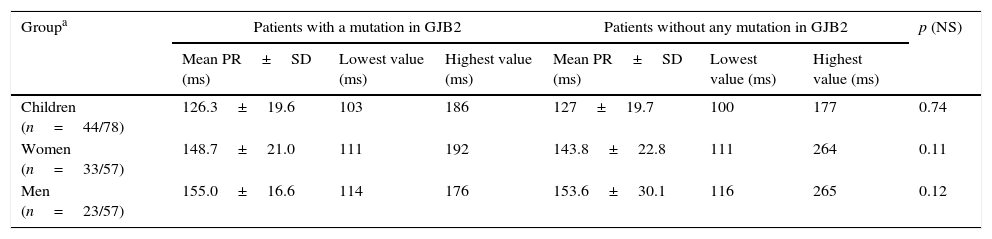
Findings of PR interval in the ECG study population.
| Groupa | Patients with a mutation in GJB2 | Patients without any mutation in GJB2 | p (NS) | ||||
|---|---|---|---|---|---|---|---|
| Mean PR±SD (ms) | Lowest value (ms) | Highest value (ms) | Mean PR±SD (ms) | Lowest value (ms) | Highest value (ms) | ||
| Children (n=44/78) | 126.3±19.6 | 103 | 186 | 127±19.7 | 100 | 177 | 0.74 |
| Women (n=33/57) | 148.7±21.0 | 111 | 192 | 143.8±22.8 | 111 | 264 | 0.11 |
| Men (n=23/57) | 155.0±16.6 | 114 | 176 | 153.6±30.1 | 116 | 265 | 0.12 |
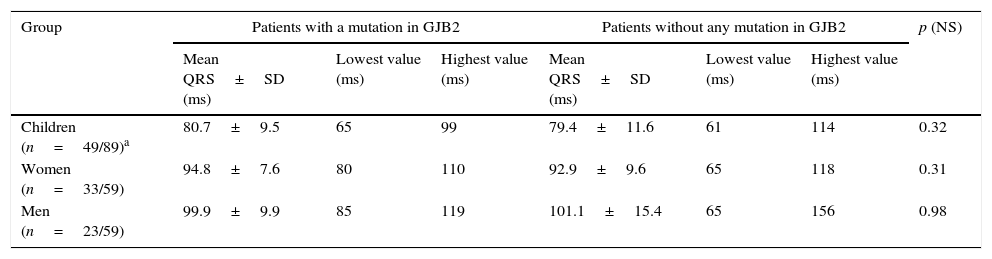
Findings of QRS complex in the ECG study population.
| Group | Patients with a mutation in GJB2 | Patients without any mutation in GJB2 | p (NS) | ||||
|---|---|---|---|---|---|---|---|
| Mean QRS±SD (ms) | Lowest value (ms) | Highest value (ms) | Mean QRS±SD (ms) | Lowest value (ms) | Highest value (ms) | ||
| Children (n=49/89)a | 80.7±9.5 | 65 | 99 | 79.4±11.6 | 61 | 114 | 0.32 |
| Women (n=33/59) | 94.8±7.6 | 80 | 110 | 92.9±9.6 | 65 | 118 | 0.31 |
| Men (n=23/59) | 99.9±9.9 | 85 | 119 | 101.1±15.4 | 65 | 156 | 0.98 |
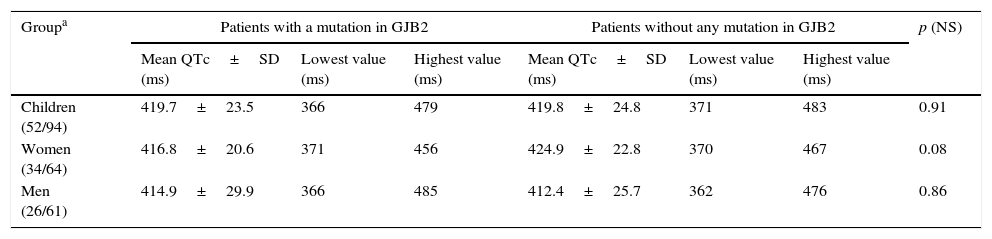
Findings of QTc interval in the ECG study population.
| Groupa | Patients with a mutation in GJB2 | Patients without any mutation in GJB2 | p (NS) | ||||
|---|---|---|---|---|---|---|---|
| Mean QTc±SD (ms) | Lowest value (ms) | Highest value (ms) | Mean QTc±SD (ms) | Lowest value (ms) | Highest value (ms) | ||
| Children (52/94) | 419.7±23.5 | 366 | 479 | 419.8±24.8 | 371 | 483 | 0.91 |
| Women (34/64) | 416.8±20.6 | 371 | 456 | 424.9±22.8 | 370 | 467 | 0.08 |
| Men (26/61) | 414.9±29.9 | 366 | 485 | 412.4±25.7 | 362 | 476 | 0.86 |
In addition, we compared parameters in patients with profound HL caused by a GJB2 mutation homozygous genotype c.[35delG];[(35delG)] and in those with a heterozygous genotype [GJB2:c.35delG];[GJB6:del(GJB6-D13S1830)] versus all other patients with other types of HL. The analysis revealed no significant differences (Table 6).
Findings of PR interval, QRS complex, and QTc interval in 2 groups. Group A, patients with homozygous genotype c.[35delG];[(35delG)] and heterozygous genotype [GJB2:c.35delG];[GJB6:del(GJB6-D13S1830)]; group B, all other patients (with other heterozygous genotype and without any mutation in GJB2).
| Children (n=31/90)a | Women (n=20/70) | Men (n=15/58) | |
|---|---|---|---|
| Mean PR±SD (ms) in group A | 122.55±18.51 | 144.1±22.98 | 153.8±15.07 |
| Mean PR±SD (ms) in group B | 128.02±25.38 | 146.3±25.74 | 154.05±25.55 |
| p | 0.0633 | 0.3934 | 0.1174 |
| Children (n=33/104) | Women (n=20/72) | Men (n=15/60) | |
|---|---|---|---|
| Mean QRS±SD (ms) in group A | 79.81±9.81 | 94.05±7.91 | 98.6±10.18 |
| Mean QRS±SD (ms) in group B | 79.87±13.2 | 93.24±13.17 | 101.27±13.88 |
| p | 0.4528 | 0.4124 | 0.4577 |
| Children (n=36/111) | Women (n=20/79) | Men (n=17/74) | |
|---|---|---|---|
| Mean QTc±SD (ms) in group A | 418.33±20.4 | 417.3±18.45 | 412.41±32.65 |
| Mean QTc±SD (ms) in group B | 420.5±24.16 | 423.74±24.07 | 414.47±24.16 |
| p | 0.4462 | 0.0908 | 0.4594 |
Finally, we analyzed the incidence of ECG abnormalities in the group of patients with a mutation in GJB2 and those without any mutation in this gene. The abnormalities in the group of patients with a mutation were: atrial rhythm (2 patients), sinus bradycardia (n=12), short time atrioventricular conduction (n=3), left anterior fascicular block (n=1), a QTc interval above the norm for age and sex (n=10), and signs of myocardial inferolateral wall necrosis associated with prolonged QTc (n=1). In the group of patients without any mutation, abnormalities were: sinus bradycardia (n=25), atrial rhythm (n=3), wandering atrial pacemaker (n=1), short time atrioventricular conduction (n=2), first degree atrioventricular block (n=5), incomplete right bundle branch block (n=1), left anterior fascicular block (n=1), complete bundle branch block (n=7), intraventricular conduction disturbances (n=3), signs of left ventricular hypertrophy (n=3), signs of myocardial ischemia (n=2), and prolonged QTc (n=23). In one boy, there was supraventricular rhythm with a prolonged atrioventricular conduction time, nonspecific intraventricular conduction disturbances, and signs of right ventricular hypertrophy.
In the group of patients with a GJB2 mutation and prolonged QTc (n=10), the mean QTc interval was 463.2±15.6ms, whereas in patients with prolonged QTc and without any mutation (n=23), the mean QTc interval was 460.3±12.8ms. This difference was also not significant (p=0.69).
DiscussionOur study acquired ECGs from 346 patients with HL who were tested for mutations in GJB2 and GJB6 genes. There is a general lack of data on detailed ECG parameters in similarly large populations. There have been reports of normal ECGs performed on 11 patients aged 9–20 years in 5 families with HL associated with GJB2 mutations.18 In addition, another study19 described 270 children with sensorineural HL of which 143 underwent an ECG: abnormalities were detected in 10 (7%), 3 had prolonged QT interval (1 had multiple atrial and ventricular septal defects and 2 were both diagnosed with Jervell and Lange–Nielsen syndrome), and other reported abnormalities included sinus bradycardia, right bundle branch block, and left ventricular hypertrophy. Of 220 children in the study group, 33 (15%) had mutations in both alleles of the GJB2 gene.
Our study is the first to our knowledge to describe ECG parameters such as PR interval and QRS complex in a large group of patients with HL. GJB2 gene mutations cause HL in 20–60% of Caucasian children. The prevalence of GJB2 mutations in the European population is highest in Mediterranean countries and lowest in the north of the continent. It is estimated that the c.35delG mutation is carried by 1–3% of the European population.20,21 The most common GJB2 genotype occurring in European patients with HL is the homozygous genotype c.[35delG];[(35delG)].20,21 In our study group of patients with HL, this genotype was also the most common. Furthermore, we have shown that among the population studied there were also other mutations, although they were rare. One rare mutation, the c.167delT reported by Dong et al.22 should be noted, however, as this mutation is uncommon in Europe generally but much more frequent in Ashkenazi Jews.23 In contrast, the most common mutation in Japanese is c.235delC, where its prevalence is 1–2.1%.24
In addition to connexin 26, a similar biological role in the inner ear is also contributed by connexin 30, encoded by the GJB6 gene, whose locus is located in the immediate vicinity of the GJB2 gene on chromosome 13.25,26 Deletion of 342kb encompassing the GJB6 gene has been described, particularly in Spanish patients. Further studies have shown that the phenotypic effects of mutations in both genes are identical, and a compound heterozygous genotype [GJB2:c.35delG];[GJB6:del(GJB6-D13S1830)] is characterized by hearing loss.16
Our assessment of electrocardiographic parameters in patients with HL caused by GJB2 mutation has shown that atrioventricular conduction (defined by PR interval), intraventricular conduction time (defined by QRS duration), and the QTc interval did not differ significantly in comparison to patients with HL who did not have any mutations in this gene.
Limitations of the studyOur study did contain some limitations. Different phenotypic expressions are the result of various GJB2 mutations. Since the described GJB2 gene mutations that result in HL are recessive, we compared parameters from a group of patients with a homozygous c.[35delG];[(35delG)] genotype and a heterozygous [GJB2:c.35delG];[GJB6:del(GJB6-D13S1830)] genotype with all other patients. The results of such an analysis are limited by the small number of people with a homozygous c.[35delG];[(35delG)] genotype, and there were only 2 children with heterozygous [GJB2:c.35delG];[GJB6:del(GJB6-D13S1830)] genotype giving the phenotypic effect of deafness.
In the study group, the presence of heart defects has not been ruled out by a reliable test (e.g. echocardiography), and neither has the effect of certain medications which might have influenced electrocardiographic parameters. The study group was selected by screening of people with HL who did not report symptoms of heart disease or did not take drugs, producing a group of apparently healthy people who had HL.
ConclusionIn patients with HL, no association was found between ECG parameters and the presence of GJB2 mutations encoding connexin 26.
Conflicts of interestThe authors declare no conflicts of interest.
This work was supported by the National Center for Science [grant number NN 402526739].
Please cite this article as: Sanecka A, Biernacka EK, Sosna M, Mueller-Malesinska M, Ploski R, Skarzynski H, et al. Evaluation of electrocardiographic parameters in patients with hearing loss genotyped for the connexin 26 gene (GJB2) mutations. Braz J Otorhinolaryngol. 2017;83:176–82.
Peer Review under the responsibility of Associação Brasileira de Otorrinolaringologia e Cirurgia Cérvico-Facial.