Postural instability is one of the most disabling features of Parkinson's disease.
ObjectiveTo evaluate postural balance in Parkinson's disease.
MethodsThirty patients with Parkinson's disease were compared with controls using Tetrax™ interactive balance system posturography.
ResultsFor different positions, patients with Parkinson's disease showed a significantly higher weight distribution index, fall index, Fourier transformation at low-medium frequencies (F2–F4), and significantly lower right/left and toe/heel synchronization versus controls.
ConclusionPostural imbalance in Parkinson's disease patients is characterized by the abnormalities of weight distribution index, synchronization index, Fourier transformation index, and fall index as measured by Tetrax™ posturography.
A instabilidade postural é um dos principais problemas na doença de Parkinson.
ObjetivoAvaliar o controle postural na doença de Parkinson.
MétodoUm grupo de 30 pacientes com doença de Parkinson foi comparado com um grupo controle à posturografia estática do Tetrax Interactive Balance System (Tetrax™).
ResultadosEm diferentes condições sensoriais, houve diferenças significantes entre os dois grupos, tendo sido encontrados nos parkinsonianos valores maiores do índice de distribuição de peso, do índice de risco de queda e da faixa de frequência F2-4 e valores menores da sincronização da oscilação postural direito-esquerda e dedos/calcanhares.
ConclusãoO comprometimento do controle postural em pacientes com doença de Parkinson é caracterizado por alterações na distribuição de peso, na sincronização da oscilação postural direita/esquerda e dedos/calcanhares, nas faixas de frequência de oscilação postural e no índice de risco de queda à posturografia do Tetrax™.
Postural balance can be defined as the ability of human beings to stand up straight and perform movements with no oscillations or falls. Its maintenance is determined by the integration in the central nervous system of information originating from the vestibular, visual, and proprioceptive systems that trigger eye and spinal reflexes.1–4 The vestibular-ocular reflex (VOR) generates eye movements, promoting the stabilization of gaze during head movement; the vestibulospinal reflex (VSR) generates compensatory body movements in order to maintain head and postural stability.5
Parkinson's disease is a chronic and progressive degenerative disorder of the central nervous system. It affects all age groups, but is most commonly found in the elderly population. Parkinson's disease can be considered the second most common senile neurodegenerative disease, affecting approximately 1% to 2% of the population above 65 years of age,6 and occurs in different races and social classes in both genders, but is prevalent in males.7
Parkinson's disease is characterized by rigidity, bradykinesia, micrography, mask-like facial expression, postural changes, and resting tremor. Postural changes include lack of balance reaction, adopting the posture in flexion, and decreased trunk rotation.8 It is considered that the fall risk in Parkinson's disease varies between 38% and 68%, and that recurrent falls occur more often in the later stages of the disease.9
The pathophysiology of Parkinson's disease is a progressive loss of cells in the substantia nigra of the midbrain. The degeneration of neurons in the substantia nigra results in decreased production of dopamine, with depigmentation of this structure.7,8
The etiology remains unknown, and it is believed that the pathogenic mechanisms involved are multifactorial: oxidative stress, mitochondrial abnormalities, excitotoxicity, glial and inflammatory factors, environmental neurotoxins, genetic factors, and brain aging.10,11
Patients with Parkinson's disease exhibit more difficulty in executing simultaneous movements and sequencial tasks versus simple tasks, requiring the complete execution of one movement before starting the next.8
Postural instability is a major problem in Parkinson's disease; it increases the frequency of fall episodes and their consequences, and the likelihood of occurrence of falls increases according to the extent and duration of the disease.8,12
Progression of the disease leads to an impairment of gait called festination, characterized by decreased speed and shortening of the stride, as if the person were chasing his own center of gravity, with a tendency to tip over forward. Festinant gait may be caused by a change of pressure and mass centers, resulting in a reduction of the responses of balance, or as a result of changes in gait kinematics.
Changes in gait kinematics include changes in joint excursion and in hip flexion, which can modify the excursion of the heel. Instead of a heel-toe progression, the patient makes contact with the ground with flat feet; or, with the advance of the disease, there is a heel-toe progression, significantly compromising the gait.8,13
Posturography measures postural instability, assists in the analysis of the functional aspects of whatever dysfunction causes the body imbalance,8,14,15 complements conventional vestibular tests for diagnosis, and is relevant to the staging, treatment, and prognosis of Parkinson's disease.16,17 It may also identify early signs of balance impairment in different conditions, such as with eyes open, eyes closed, and on unstable surfaces.8,18
Research has demonstrated that healthy individuals have better postural control and a higher stability limit than patients with Parkinson's disease in the on period, a phase where the patient is under the influence of anti-Parkinsonian medication and presents better motor performance, and in the off period, where there is no effect of medication and consequently a worsening of symptoms; Parkinson patients perform better in the on period than in the off period.19,20
Most posturographic devices in current use assume that the mechanisms involved in postural control can be measured by analyzing the postural oscillation manifested by a shifting of the center of gravity or pressure, while the subject remains standing on a platform sensitive to pressure.
In Tetrax™ (Sunlight Medical Ltd.) interactive balance system posturography, created by Kohen-Raz with four platforms, postural control is investigated through the difference in pressure on each platform. This equipment makes it possible to obtain and separately compare the values of forefoot and rearfoot (toes and heel) of each foot, and of each heel with the contralateral forefoot.21
The body balance of healthy individuals was analyzed with Tetrax™ in different sensory conditions (eyes open or eyes closed and with head turned 45° to the right or to the left, or with a tilt of 30° forward or backward, on a firm or unstable surface), with reference to the general stability index, weight distribution index, synchronization to the right/left, synchronization of toes/heel, and fall risk.22
This research was motivated by the authors’ finding that only two studies used Tetrax™ in patients with Parkinson's disease,23,24 and by the need to more broadly assess the postural control of patients with this disease.
ObjectiveThis study aimed to assess the postural control in patients with Parkinson's disease.
MethodsThis clinical, cross-sectional study with a consecutive sample was initiated after a review and approval by the ethics committee on research with human subjects under No. 1415-11. All volunteers were evaluated between 2011 and 2012 and were informed of the procedures that would be performed; in addition, these volunteers signed an informed consent allowing their participation in the study and subsequent publication of the results.
Thirty male and female patients with neurological diagnosis of idiopathic Parkinson's disease (experimental group), classified in stages I–III of the Hoehn and Yahr Scale, were included.25 The diagnosis was based on the criteria of the Brain Bank for Neurological Diseases, National Hospital for Neurology and Neurosurgery, in London, which requires the presence of bradykinesia and at least one of the three cardinal signs of Parkinson's disease: tremor at rest, muscle rigidity, and postural instability.26
The control group was matched for age and gender in relation to the experimental group, and included 29 volunteers from the community. Inclusion criteria for this group were the absence of neurological diseases and body imbalance, no history of vestibular and/or auditory symptoms, and a vectoelectronystagmography vestibular examination within normal reference parameters.
The exclusion criteria included patients who showed changes in the external and/or middle ear those with psychiatric disorders, history of ear; surgery, inability to understand and follow simple verbal commands, inability to remain in the upright position independently; or who had severe visual impairment not compensated with corrective lenses, orthopedic disorders resulting in limited movement, used prosthetic legs, or who have received body balance rehabilitation in the last six months.
Patients with Parkinson's disease were assessed during the on period, between 40min and 2h after administration of levodopa, when they exhibited better motor performance.27
Static Tetrax™ posturography includes a specific program installed on a computerized platform composed of four independent and integrated platforms (A–B–C–D), placed on level ground, uncarpeted, with a handrail and a foam mat.
Patients placed their toes and heels on the four platforms (A – left heel; B – left toes; C – right heel, D – right toes) with arms outstretched alongside the body, and were instructed to maintain an upright, stable, and immobile posture for 32s for each of the eight sensory conditions: “face forward, eyes open, looking at a target on the wall opposite the platform on firm surface” (NO); “face forward, eyes closed on a firm surface” (NC); “eyes closed, 45° of head rotation to the right on a firm surface” (HR); “eyes closed, 45° of head rotation to the left on a firm surface” (HL); “eyes closed, head tilted 30° backward on a firm surface” (HB); “eyes closed, head tilted 30° forward on a firm surface” (HF), “face forward, eyes open, looking at a target on the wall opposite the platform on a unstable surface on a cushion” (PO); and “face forward, eyes closed on an unstable surface on a cushion” (PC).
The posturography with Tetrax™ evaluated the following parameters: stability index, weight distribution index, left/right and toes/heel synchronization index, postural oscillation frequency (F1, F2–F4, F5–F6, and F7–F8), and fall index.28
The stability index, regardless of weight and height, indicates the overall stability and the ability to make postural changes. This index assesses the amount of oscillation on the four platforms of Tetrax™.28
The weight distribution index, expressed as a percentage, is measured by comparing the deviations from the weight distribution on each platform with respect to an expected average value of 25%.28
The synchronization index between the heel and toes of each foot (AB, CD), between the two heels and toes of both feet (AC, BD), and between the heel of one foot with the contralateral toes (AD, BC) measures the coordination between the lower limbs and the symmetry in the distribution of weight in each condition.28
The frequency of postural oscillation, measured by Fourier transformation, determines the intensity of postural oscillation in a variable spectrum between 0.01 and 3.0Hz. Tetrax™ subdivides the spectrum of postural oscillation in four frequency ranges: low (F1), below 0.1Hz; medium-low (F2–F4), between 0.1 and 0.5Hz; medium-high (F5–F6), between 0.5 and 1.0Hz; and high (F7–F8), above 1.0Hz.28
The fall index, expressed as a percentage and ranging between 0 and 100, analyzes the results of Tetrax™ parameters in the eight conditions. A value between 0% and 36% is judged as mild risk; a value between 37% and 58%, as moderate risk; and between 59% and 100%, as high risk. The higher the score, the greater the risk of occurrence of a fall.28
All data were submitted to descriptive statistics for sample characterization. Levene's test was used to analyze the equivalence of variances with respect to age, and the chi-squared test was used for the analysis of homogeneity of genres between the control and experimental groups. The Shapiro–Wilk test was used to verify the normality of the variables.
In the comparative analysis of experimental and control groups, the nonparametric Mann–Whitney test was used for the overall stability index, for left-right and toes/heel synchronization in the eight sensory conditions, and for the weight distribution index in the “NO”, “NC”, “PO”, “HR”, “HL”, and “HB” conditions. Student's t-test was used for independent samples, regarding age, fall index, postural oscillation frequencies (F1, F2–F4, F5–F6, and F7–F8) in all conditions, and weight distribution index in “PC” and “HF” conditions. Data were presented as mean±standard deviation. The level of significance was set at p<0.05. Predictive Analytics Software (PASW, version 18.0) was used for calculations.
ResultsA total of 59 patients were evaluated: 30 in the experimental group, of whom 18 (60.0%) were male and 12 (40.0%) female, and 29 in the control group, of whom 11 (37.9%) were male and 18 (62.1%) female. The mean age (±SD) of the group with Parkinson's disease was 59.8±10.3 years. The mean age (±SD) of the control group was 58.9±9.8 years. The groups were homogeneous with respect to gender (p=0.151) and age (p=0.488).
On average, the risk of occurrence of a fall was moderate (mean±SD=42.1±25.5) in patients with Parkinson's disease and mild (mean±SD=22.7±13.7) in the control group. The group with Parkinson's disease showed a higher risk of fall versus the control group, with a statistically significant difference (p=0.001).
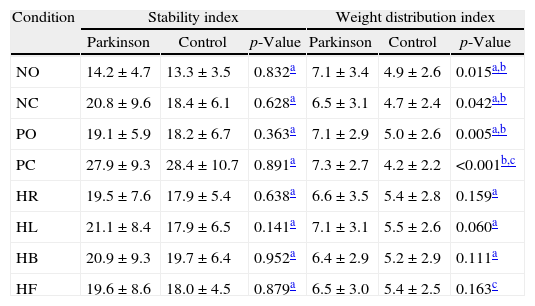
Table 1 presents a comparative analysis of the stability index and of the weight distribution index of both groups on Tetrax™. There was no statistically significant difference between the groups regarding the stability index in the studied conditions. The weight distribution index was higher in patients with Parkinson's than in the control group in all conditions of Tetrax™, with a statistically significant difference in “NO”, “NC”, “PO”, and “PC” conditions.
Analysis of the stability index and weight distribution index in the eight conditions of the Tetrax™ interactive balance system in 29 control subjects and 30 patients with Parkinson's disease.
| Condition | Stability index | Weight distribution index | ||||
| Parkinson | Control | p-Value | Parkinson | Control | p-Value | |
| NO | 14.2±4.7 | 13.3±3.5 | 0.832a | 7.1±3.4 | 4.9±2.6 | 0.015a,b |
| NC | 20.8±9.6 | 18.4±6.1 | 0.628a | 6.5±3.1 | 4.7±2.4 | 0.042a,b |
| PO | 19.1±5.9 | 18.2±6.7 | 0.363a | 7.1±2.9 | 5.0±2.6 | 0.005a,b |
| PC | 27.9±9.3 | 28.4±10.7 | 0.891a | 7.3±2.7 | 4.2±2.2 | <0.001b,c |
| HR | 19.5±7.6 | 17.9±5.4 | 0.638a | 6.6±3.5 | 5.4±2.8 | 0.159a |
| HL | 21.1±8.4 | 17.9±6.5 | 0.141a | 7.1±3.1 | 5.5±2.6 | 0.060a |
| HB | 20.9±9.3 | 19.7±6.4 | 0.952a | 6.4±2.9 | 5.2±2.9 | 0.111a |
| HF | 19.6±8.6 | 18.0±4.5 | 0.879a | 6.5±3.0 | 5.4±2.5 | 0.163c |
NO, eyes open on a firm surface; NC, eyes closed on a firm surface; PO, eyes open on an unstable surface; PC, eyes closed on an unstable surface; HR, eyes closed with head rotation to the right on a firm surface; HL, eyes closed with head rotation to the left on a firm surface; HB, eyes closed, head tilted backward 30° on a firm surface; HF, eyes closed, head tilted forward 30° on a firm surface.
Values=mean±standard deviation.
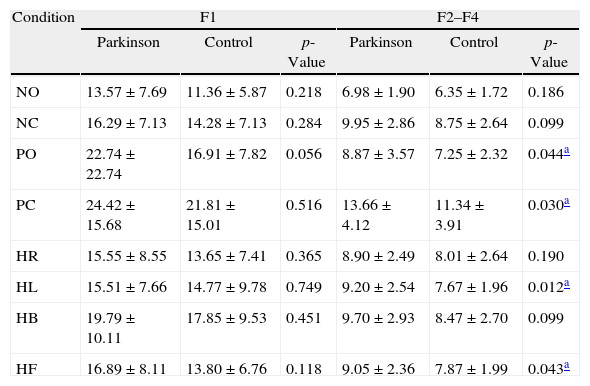
Table 2 shows the comparative analysis of the postural oscillation frequency ranges (F1, F2–F4, F5–F6, and F7–F8) in the control and experimental groups in the eight sensory conditions of Tetrax™. The group with Parkinson's disease had higher values versus the control group in all frequency ranges and in all sensory conditions.
Comparative analysis of Fourier frequency ranges in the eight conditions of the Tetrax™ interactive balance system in 29 control subjects and 30 patients with Parkinson's disease.
| Condition | F1 | F2–F4 | ||||
| Parkinson | Control | p-Value | Parkinson | Control | p-Value | |
| NO | 13.57±7.69 | 11.36±5.87 | 0.218 | 6.98±1.90 | 6.35±1.72 | 0.186 |
| NC | 16.29±7.13 | 14.28±7.13 | 0.284 | 9.95±2.86 | 8.75±2.64 | 0.099 |
| PO | 22.74±22.74 | 16.91±7.82 | 0.056 | 8.87±3.57 | 7.25±2.32 | 0.044a |
| PC | 24.42±15.68 | 21.81±15.01 | 0.516 | 13.66±4.12 | 11.34±3.91 | 0.030a |
| HR | 15.55±8.55 | 13.65±7.41 | 0.365 | 8.90±2.49 | 8.01±2.64 | 0.190 |
| HL | 15.51±7.66 | 14.77±9.78 | 0.749 | 9.20±2.54 | 7.67±1.96 | 0.012a |
| HB | 19.79±10.11 | 17.85±9.53 | 0.451 | 9.70±2.93 | 8.47±2.70 | 0.099 |
| HF | 16.89±8.11 | 13.80±6.76 | 0.118 | 9.05±2.36 | 7.87±1.99 | 0.043a |
| Condition | F5–F6 | F7–F8 | ||||
| Parkinson | Control | p-Value | Parkinson | Control | p-Value | |
| NO | 2.85±1.13 | 2.67±0.91 | 0.508 | 0.44±0.16 | 0.43±0.17 | 0.695 |
| NC | 4.10±1.91 | 3.33±1.10 | 0.064 | 0.69±0.28 | 0.60±0.34 | 0.308 |
| PO | 3.73±1.32 | 3.61±1.49 | 0.742 | 0.64±0.21 | 0.60±0.20 | 0.498 |
| PC | 4.94±1.74 | 4.97±1.64 | 0.945 | 0.88±0.35 | 0.91±0.38 | 0.762 |
| HR | 3.49±1.32 | 3.36±1.07 | 0.679 | 0.63±0.31 | 0.57±0.22 | 0.390 |
| HL | 3.94±1.69 | 3.33±1.23 | 0.119 | 0.67±0.25 | 0.59±0.26 | 0.218 |
| HB | 3.75±1.54 | 3.68±1.27 | 0.854 | 0.67±0.34 | 0.69±0.24 | 0.883 |
| HF | 3.75±1.82 | 3.56±1.07 | 0.621 | 0.62±0.27 | 0.60±0.21 | 0.730 |
NO, eyes open on a firm surface; NC, eyes closed on a firm surface; PO, eyes open on an unstable surface; PC, eyes closed on an unstable surface; HR, eyes closed with head rotation to the right on a firm surface; HL, eyes closed with head rotation to the left on a firm surface; HB, eyes closed, head tilted 30° backward on a firm surface; HF, eyes closed, head tilted forward 30° on firm surface.
F1, F2–F4, F5–F6, F7–F8, postural oscillation frequency ranges.
Values are shown as mean±standard deviation.
Student's t-test.
There was a statistically significant difference between the groups in the F2–F4 range in “PO”, “PC”, “HL”, and “HF” conditions. There was no statistically significant difference in “NO”, “NC”, “HR”, and “HB” conditions.
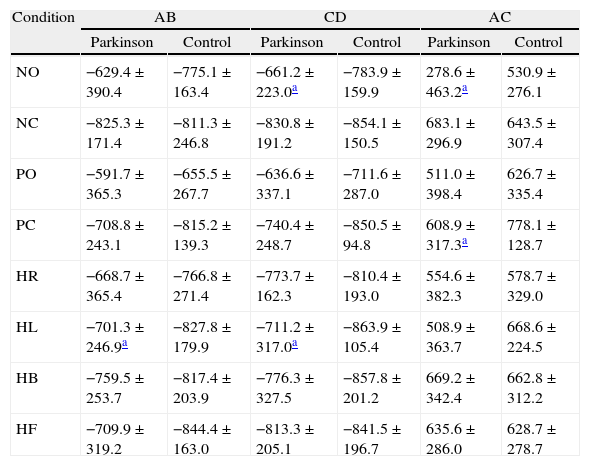
Table 3 shows the comparative analysis of the right/left and toes/heel synchronization index of the control and experimental groups in the eight sensory conditions of Tetrax™.
Comparative analysis of synchronization indexes in the eight conditions of the Tetrax™ interactive balance system in 29 control subjects and 30 patients with Parkinson's disease.
| Condition | AB | CD | AC | |||
| Parkinson | Control | Parkinson | Control | Parkinson | Control | |
| NO | −629.4±390.4 | −775.1±163.4 | −661.2±223.0a | −783.9±159.9 | 278.6±463.2a | 530.9±276.1 |
| NC | −825.3±171.4 | −811.3±246.8 | −830.8±191.2 | −854.1±150.5 | 683.1±296.9 | 643.5±307.4 |
| PO | −591.7±365.3 | −655.5±267.7 | −636.6±337.1 | −711.6±287.0 | 511.0±398.4 | 626.7±335.4 |
| PC | −708.8±243.1 | −815.2±139.3 | −740.4±248.7 | −850.5±94.8 | 608.9±317.3a | 778.1±128.7 |
| HR | −668.7±365.4 | −766.8±271.4 | −773.7±162.3 | −810.4±193.0 | 554.6±382.3 | 578.7±329.0 |
| HL | −701.3±246.9a | −827.8±179.9 | −711.2±317.0a | −863.9±105.4 | 508.9±363.7 | 668.6±224.5 |
| HB | −759.5±253.7 | −817.4±203.9 | −776.3±327.5 | −857.8±201.2 | 669.2±342.4 | 662.8±312.2 |
| HF | −709.9±319.2 | −844.4±163.0 | −813.3±205.1 | −841.5±196.7 | 635.6±286.0 | 628.7±278.7 |
| Condition | BD | AD | BC | |||
| Parkinson | Control | Parkinson | Control | Parkinson | Control | |
| NO | 621.8±348.1 | 734.8±167.8 | −732.8±367.7 | −839.9±129.7 | −740.5±384.6 | −818.2±192.5 |
| NC | 822.1±161.2 | 847.9±108.4 | −894.6±143.3 | −870.1±167.3 | −902.0±109.6 | −898.9±81.0 |
| PO | 561.5±325.1 | 599.3±288.5 | −863.4±172.9 | −908.3±98.1 | −875.7±148.0* | −911.2±99.9 |
| PC | 737.4±173.2 | 802.0±133.6 | −901.8±106.2 | −948.4±34.0 | −905.2±80.0* | −946.7±41.4 |
| HR | 713.6±238.4 | 791.2±220.9 | −850.6±155.7 | −850.5±149.2 | −891.4±134.8 | −889.4±93.2 |
| HL | 734.8±218.1 | 805.0±169.3 | −843.6±166.6 | −872.8±130.3 | −844.5±251.0 | −872.3±118.3 |
| HB | 760.8±244.9 | 823.1±189.2 | −906.7±94.0 | −869.3±124.9 | −871.2±283.0 | −889.3±104.2 |
| HF | 746.9±295.9 | 843.1±146.6 | −860.3±134.5 | −878.8±86.0 | −915.6±50.9 | −871.8±134.2 |
AB, synchronization index between platforms regarding left toes and heel; CD, synchronization index between right toes and heel; AC, synchronization index between both heels; BD, synchronization index between both forefeet; AD, synchronization index between left and right toes; BC, synchronization index between left toes and right heel; NO, eyes open on a firm surface; NC, eyes closed on a firm surface; PO, eyes open on an unstable surface; PC, eyes closed on an unstable surface; HR, eyes closed with head rotation to the right on a firm surface; HL, eyes closed with head rotation to the left on a firm surface; HB, eyes closed, head tilted backward 30° on a firm surface; HF, eyes closed, head tilted forward 30° on a firm surface.
Values shown are mean±standard deviation.
Mann–Whitney test.
There was a statistically significant difference between the groups, with lower values in the group of Parkinson's patients in synchronizations “CD” (p=0.018) and “AC” (p=0.033) in “NO” condition; in synchronization “BC” (p=0.017) in “PO” condition; in synchronizations “BC” (p=0.035) and “AC” (p=0.049) in “PC” condition; in synchronizations “AB” (p=0.041) and “CD” (p=0.022) in “HL” condition. The difference between the synchronization indexes AB “exhibited” borderline significance (p=0.05) in “PC”. There was no significant difference between the groups in synchronizations AB, CD, AC, BD, AD, and BC (p>0.05) in “NC”, “HR”, “HB” and “HF” conditions.
DiscussionThe findings of the static Tetrax™ posturography in the group of patients with Parkinson's disease were compared to those obtained in the control group. There are limitations in the quantitative comparison of results with those of other posturographies, since Tetrax™ employs different parameters and methods of evaluation.
Tetrax™ provides the fall index through a mathematical algorithm which is based on the patient's performance in the various evaluated parameters.28 In the present study, the risk of occurrence of a fall was, on average, mild in the control group and moderate in the Parkinson's disease group; it was significantly higher in patients with Parkinson's disease, in agreement with the findings of a survey also conducted with Tetrax™ in mild or moderate Parkinson's disease, in accordance with the Hoehn-Yahr scale (modified),23 but in disagreement with another study on patients in the early stages of Parkinson's disease, which identified no significant difference versus control group.24
Postural instability is a major problem in Parkinson's disease, and the occurrence of falls is one of its most serious complications. The percentage of patients suffering falls varies between 38% and 68%.9 With disease progression, the frequency of falls increases.29 The identification of the fall risk can lessen the risk for the occurrence of this event in the future, through early intervention.30 Most studies investigating the fall risk use data from questionnaires or in patient reports to predict future falls. However, patients often do not accurately remember the details of previous falls, of the interval between the events, and, importantly, whether they have occurred over a long period of time.31
The values of the weight distribution index were significantly higher in patients with Parkinson's disease, compared to that in the control group, in four of the eight evaluated sensory conditions: “NO”, “NC”, “PO”, and “PC”. Similar to the present findings, another study23 employing Tetrax™ showed a significant difference in the “NO and “NC” sensory conditions; but unlike the present study results, significantly higher values were observed in “HR”, “HB” (head backwards), and HF (“head forward”) conditions. The higher the weight distribution index, the greater the difficulty in maintaining posture.28
The stability index of the group with Parkinson's disease was similar to that of the control group in all the evaluated sensory conditions. However, compared with the control group, in Parkinson's disease it was reported that the stability index in Tetrax™ was higher only in “NC” condition, with no significant difference in other conditions.23 With other types of static posturography, some authors have demonstrated in Parkinsonian patients a significantly greater oscillation area in “eyes open and eyes closed on a firm surface” conditions,32–34 while others have observed no significant difference.18,35
The values of left and right toes/heels synchronization index of the control group and of the group with Parkinson's disease were symmetrical in the eight sensory conditions. However, the group with Parkinson's disease had significantly lower values in synchronizations “CD” and “AC” in “NO” condition; “BC” in “PO” condition; “BC” and “AC” in “PC condition”; and “AB” and “CD” in “HL” condition. Significantly lower values were reported only in AC synchronization, in HB condition.23 The symmetry of the values of synchronizations could be due to adequate compensatory mechanisms and to the simultaneous activation of the parallel plates.28
Regarding the postural oscillation frequency ranges (F1, F2–F4, F5–F6, and F7–F8), the group with Parkinson's disease showed significantly higher values versus the control group only in the range F2–F4 in “PO”, “PC”, “HL” and “HF” conditions, suggesting peripheral vestibular dysfunction.28
Both groups showed dominance of oscillation at low frequencies, indicating visual preference to maintain postural control.28 Significant differences between the group with Parkinson's disease and the control group were also found with Tetrax™ in F2–F4 frequency ranges in “NC”, “HL”, “HR”, “HF”, and “HB” conditions; in F5–F6 ranges in NC and HL conditions; and in the F7–F8 ranges in NC, HL, and HR conditions.23
In patients in the early stages of Parkinson's disease, Tetrax™ identified greater involvement of the central vestibular system,24 characterized by greater oscillation in the F7–F8 frequency range.28 The difference found between the groups in F2–F4 range may have been an attempt to compensate for a proprioceptive impairment. The decrease in proprioceptive information on unstable surface conditions, a restriction of movement and a rigidity to change in the head position may have hampered the maintenance of posture. The deterioration of proprioceptive information can cause postural instability.36–38
In this research, it was observed that the Tetrax™ posturography provides relevant information regarding the stability index, weight distribution index, right/left and toes/heels synchronization index, frequency ranges of postural oscillation (F1, F2–F4, F5–F6, F7–F8), and fall index in the assessment of postural control in Parkinson's disease.
The characterization of a body balance disorder in each patient with this condition may have diagnostic, therapeutic, and even preventive implications. Further research should be conducted in this area in order to better understand the relationship among postural control and the different stages of Parkinson's disease, as well as the possible role of prophylactic or therapeutic procedures for body balance rehabilitation in solving or mitigating the imbalance and in eliminating or reducing the risk of falls in these patients.
ConclusionImpaired postural control in patients with Parkinson's disease in Tetrax™ posturography is characterized by changes in distribution of weight in the synchronization of right/left and toes/heels postural oscillation, in the postural oscillation frequency ranges, and in the fall index.
FundingThis study was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Fukunaga JY, Quitschal RM, Doná F, Ferraz HB, Ganança MM, Caovilla HH. Postural control in Parkinson's disease. Braz J Otorhinolaryngol. 2014;80:508–14.