Sentinel lymph node biopsy is the gold standard procedure for head and neck cutaneous melanoma staging.
ObjectiveTo evaluate the technical aspects, positivity and prognostic effect of the cervico-facial sentinel lymph node biopsy.
MethodsRetrospective, unicentric study. From 2009 to 2014, 49 patients with cutaneous melanoma of the head and neck underwent surgery at Instituto do Câncer do Estado de São Paulo (ICESP).
ResultsOf the 49 patients, 5 had cervical metastasis at the moment of admission. Clark, Breslow and mitotic index were predictors of death. Among the 31 patients undergoing sentinel lymph node biopsy, 3 had positive sentinel lymph nodes (9.7%). Deaths were recorded in two of the cases with positive sentinel lymph nodes (66.6%), and in 5 (17.8%) of the patients with negative lymph nodes. The mean Breslow index was 11.3 mm for primary melanomas with positive sentinel lymph nodes and 4.3 mm for those with negative sentinel lymph nodes. Positivity was associated with Clark and Breslow levels. Malar location showed a protective effect on prognosis. The mean survival for patients with a mitotic index <3.5 was 181 months and 63.4 months for those with a mitotic index >3.5.
ConclusionThe frequency of positive sentinel lymph node biopsy in patients with malignant melanoma of the head and neck was lower than in other studies, although the sample consisted of individuals with advanced melanomas. The mitotic index was important for prognosis prediction.
Melanoma is the most lethal malignant skin tumor. The number of cases of the disease increases each year in Brazil and worldwide.1,2 According to the Brazilian National Cancer Institute (INCA, Instituto Nacional do Câncer) estimates, by 2020 8450 new cases of cutaneous melanoma are predicted in the country, of which 4200 may occur in men and 4250 in women.1 It has been estimated that 290,000 new cases and 61,000 deaths were recorded worldwide in 2018.1
Treatment is mainly surgical and the main prognostic indicators are: Breslow index, lymph node involvement (macro or micro-metastases), ulceration and mitotic index.3,4
The sentinel lymph node biopsy, developed by Morton in the early 1990s, became the gold standard for the staging of cutaneous melanomas, aiming to select cases for lymphadenectomy.4,5 There are peculiarities regarding the screening for sentinel lymph nodes in the head and neck region: experience shows that the use of a lesser amount of patent blue is desirable, in order to avoid tattooing by the dye6; it is not always possible to enlarge the most adequate margin in places such as the nose and periorbital region7; moreover, some authors describe a lower frequency of micro-metastases in sentinel lymph nodes for the cervicofacial region, when compared to melanomas draining to the axillary and inguinal regions.8–10
Technological advances have increased the accuracy of the results and improved the techniques used in the cervicofacial region, especially the fusion of lymphoscintigraphy and computed tomography imaging (SPECT-CT), and even minimizing the percentage of false-negative results in the sample.10 The current management provides for the screening of sentinel lymph nodes for melanomas with a Breslow index >1.0 mm (or below, if there is ulceration and/or positive mitotic index).2
The Brazilian reality implies a substantial number of patients treated by SUS who have advanced melanomas. Moreover, there are few services with integrated nuclear medicine to perform sentinel lymph node screening.11 At the Instituto do Câncer do Estado de São Paulo (ICESP), cervicofacial melanomas are treated by a multidisciplinary team, which includes the head and neck surgery team. The object of this study is an evaluation of the technical aspects, positivity and prognostic effect of sentinel lymph node screening.
MethodsThis is a retrospective study, approved by the research ethics committee (opinion N. 743,366), which reviewed the medical records of 49 patients with primary cutaneous melanoma in the head and neck region, without distant metastasis at the time of the diagnosis, undergoing surgery at ICESP by the head and neck surgical team, between 2009 and 2014. The patients were treated consecutively by the same surgeon and had a minimum follow-up time of 5 years. Cases of mucosal melanoma and primary cutaneous tumors in other topographic regions, other than head and neck, were excluded.
Data were collected on the following variables: gender, age, primary tumor site, Breslow index, level of Clark, ulceration, mitotic index, TNM staging, clinical stage, sentinel lymph node screening indication, time of follow-up, recurrence and death.
All patients underwent excisional biopsy. In cases where the procedure was previously performed at another institution, a slide review was performed. All patients underwent staging exams, including chest X-rays. For 30 patients, preoperative computed tomography was also indicated.
According to the recommendations of the NCCN (National Comprehensive Cancer Network) guidelines,2 patients with melanoma in situ underwent a 0.5 cm-margin enlargement around the previous scar. Those who had a Breslow index between 0 and 1.0 mm in the anatomopathological analysis had a 1.0 cm-margin enlargement around the previous incision. In cases where the Breslow index was between 1.0 and 2.0 mm, it was decided to expand it to between 1.0 and 2.0 cm, according to the best aesthetic and functional result and, in cases with thickness >2.0 mm, the margin was expanded by 2.0 cm. In all cases, reconstruction with skin flaps or grafts occurred at the same surgical event.
Sentinel lymph node screening was indicated in patients without detected lymph node or distant metastasis in which the Breslow index was >1.0 mm or <1.0 mm in the presence of ulceration and/or positive mitotic index. The eligible patients received an injection of radiotracer (Technetium 99; 0.5 mCi in 1 mL), the day before surgery and the image obtained by SPECT-CT was analyzed by the team at the time of surgery. The screening for micro-metastases in the removed lymph node was carried out after it was fixed in formaldehyde, processed in paraffin, and analyzed in serial sections after stained with hematoxylin-eosin and the immunohistochemical expression of HMB-45, S-100 and Melan-A was investigated, according to the protocol.2
In patients with histologically confirmed lymph node metastasis, lymph node dissection was performed according to the location of the primary tumor and the detected lymph node. A parotidectomy was performed, with preservation of the facial nerve, if the metastasis was in the parotid, followed by the dissection of levels I, II and III; posterolateral dissection, when the metastasis was retroauricular or occipital; in the case of metastasis levels I–V, a modified radical dissection was performed. The indication for radiotherapy occurred in patients with macro-metastasis identified in the dissected product. Patients diagnosed with distant metastasis during followup were treated with dacarbazine-based chemotherapy.
All statistical analyses were carried out using SPSS software, version 24.0 (SPSS Inc. Illinois, USA). The values obtained when studying the quantitative variables were organized and described using the mean and standard deviation, and for qualitative variables, the absolute and relative frequencies were used. Quantitative variables were compared using the Student's t test and normality standards were verified using the Kolmogorov–Smirnov test. For non-parametric variables, the Mann-Whitney test was used. The Chi-square test compared the qualitative variables, using Fisher’s test when necessary. Kaplan–Meier curves were constructed to disclose differences in survival analyses and the log-rank, Breslow and Tarone–Ware tests were applied when comparing them. The Receiver Operating Characteristic, or ROC curve, was produced to determine the best discriminatory value for the mitotic index. A level of statistical significance of less than 5% (p < 0.05) was adopted.
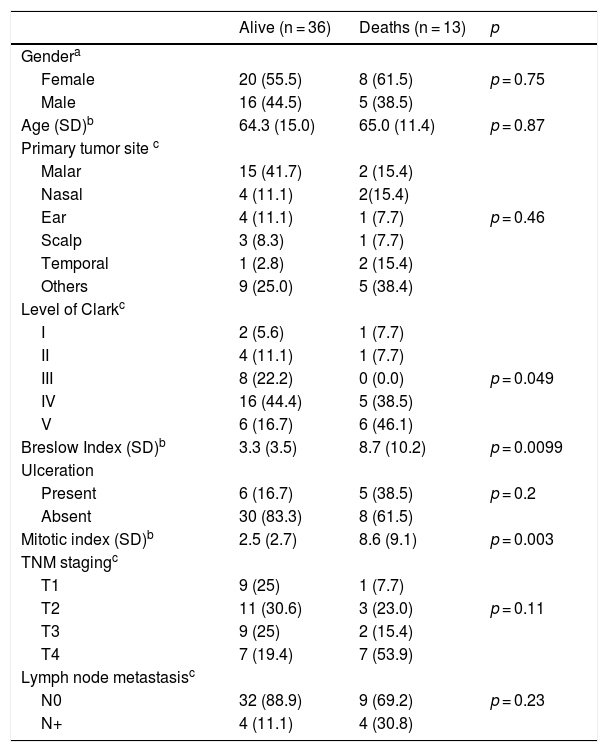
ResultsThe followup period ranged from 4 to 222 months. Of the 49 patients that were followed, 36 (73.4%) remained alive, 10 (20.4%) had recurrence and 13 (26.5%) died. Of the 10 patients with recurrence, five had pulmonary metastasis, one had a cervical lymph node recurrence, one had an axillary lymph node, one had a liver metastasis, one a mediastinal nodule and one had a local recurrence. Of the 13 who died, the cause of death was progression of melanoma in 10, and three died due to a second primary neoplasm (digestive hemorrhage due to gastric Burkitt’s lymphoma, progression of supra-glottic carcinoma and progression of colon carcinoma). Table 1 describes the distribution of demographic, clinical, anatomopathological and staging data, according to the outcome.
Distribution of cases of cutaneous malignant melanoma of the head and neck according to demographic, clinical, anatomopathological variables, and vital status at the end of the follow-up period.
| Alive (n = 36) | Deaths (n = 13) | p | |
|---|---|---|---|
| Gendera | |||
| Female | 20 (55.5) | 8 (61.5) | p = 0.75 |
| Male | 16 (44.5) | 5 (38.5) | |
| Age (SD)b | 64.3 (15.0) | 65.0 (11.4) | p = 0.87 |
| Primary tumor site c | |||
| Malar | 15 (41.7) | 2 (15.4) | |
| Nasal | 4 (11.1) | 2(15.4) | |
| Ear | 4 (11.1) | 1 (7.7) | p = 0.46 |
| Scalp | 3 (8.3) | 1 (7.7) | |
| Temporal | 1 (2.8) | 2 (15.4) | |
| Others | 9 (25.0) | 5 (38.4) | |
| Level of Clarkc | |||
| I | 2 (5.6) | 1 (7.7) | |
| II | 4 (11.1) | 1 (7.7) | |
| III | 8 (22.2) | 0 (0.0) | p = 0.049 |
| IV | 16 (44.4) | 5 (38.5) | |
| V | 6 (16.7) | 6 (46.1) | |
| Breslow Index (SD)b | 3.3 (3.5) | 8.7 (10.2) | p = 0.0099 |
| Ulceration | |||
| Present | 6 (16.7) | 5 (38.5) | p = 0.2 |
| Absent | 30 (83.3) | 8 (61.5) | |
| Mitotic index (SD)b | 2.5 (2.7) | 8.6 (9.1) | p = 0.003 |
| TNM stagingc | |||
| T1 | 9 (25) | 1 (7.7) | |
| T2 | 11 (30.6) | 3 (23.0) | p = 0.11 |
| T3 | 9 (25) | 2 (15.4) | |
| T4 | 7 (19.4) | 7 (53.9) | |
| Lymph node metastasisc | |||
| N0 | 32 (88.9) | 9 (69.2) | p = 0.23 |
| N+ | 4 (11.1) | 4 (30.8) |
SD, standard deviation.
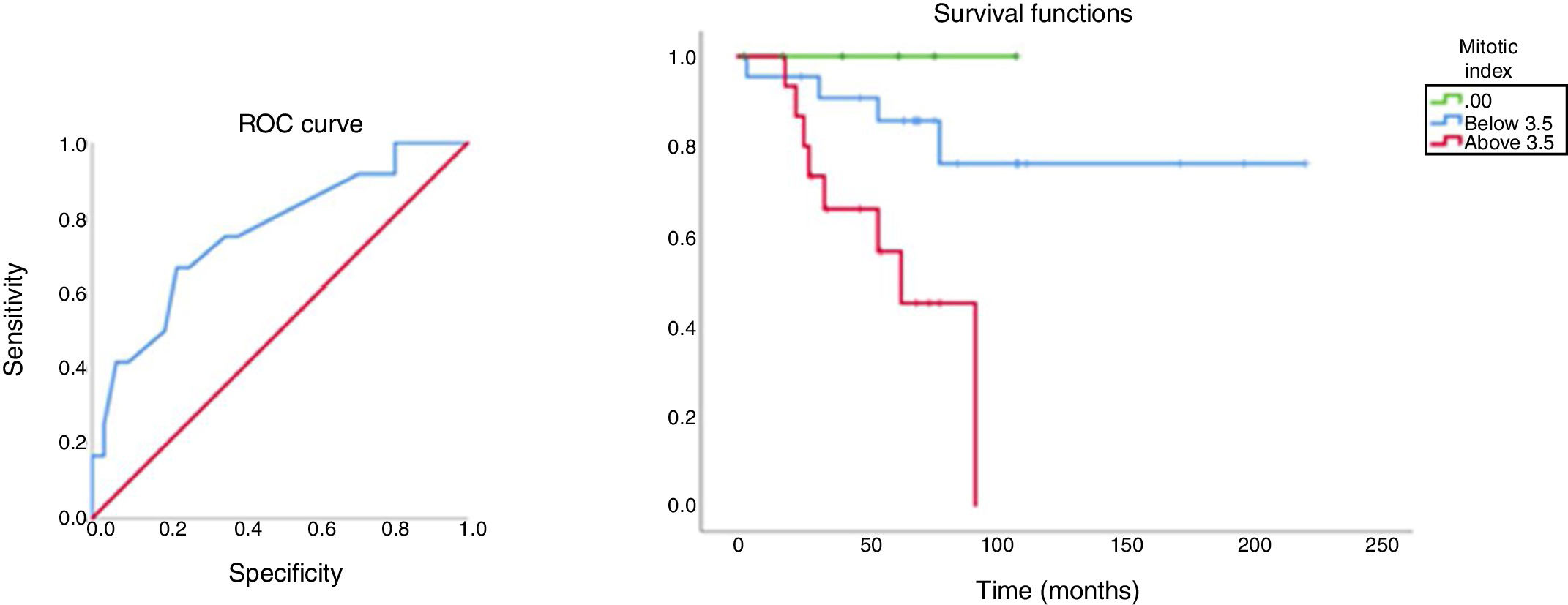
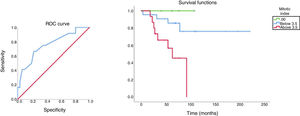
With a discriminatory value of 3.5, the analysis of survival in relation to the mitotic index and the respective ROC curve is shown in Fig. 1. The mean survival time of patients with mitotic index <3.5 was 181 months and for those with mitotic index >3.5, it was 63.4 months.
A total of 31 patients had an indication for sentinel lymph node screening (65.3%). In 20 patients, a single lymph node was removed; in seven, two lymph nodes; in three patients, three lymph nodes, and in one patient, four lymph nodes were removed. The most common site of occurrence was level Ib (15 cases), followed by level II (10 cases), parotid (5 cases), level Ia and retroauricular region (2 cases) and level V (1 case). There were three cases of positive sentinel lymph nodes, two at level Ib and one in the parotid. In two patients, the anatomopathological diagnosis was made with hematoxylin-eosin staining and one through immunohistochemistry. In one patient, dissection of the facial nerve trunk was necessary to perform the sentinel lymph node screening. This patient underwent parotidectomy and modified radical neck dissection after the anatomopathological analysis confirmed the presence of micro-metastasis.
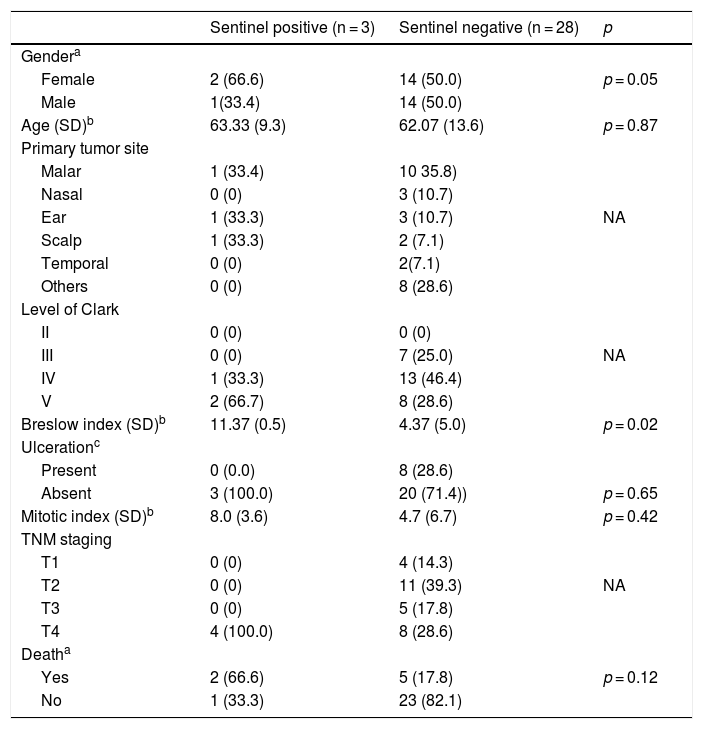
Of the three patients who had a positive sentinel lymph node, two underwent modified radical neck dissection and one underwent a parotidectomy with modified radical neck dissection. No residual disease was observed in the two modified radical neck dissections and in the dissection associated with the parotidectomy. In the posterolateral dissection, one lymph node without extracapsular extension of the 17 dissected ones showed metastasis. This patient was referred to radiotherapy, whereas the others were not treated with adjuvant treatment. Of the 28 patients with negative sentinel lymph node, 22 remained free of recurrence up to the last objective followup information; three had pulmonary metastasis, one had axillary metastasis, another had mediastinal tumor progression and the last one developed cervical metastasis 5 years after the sentinel lymph node biopsy. This patient underwent a rescue modified radical neck dissection, adjuvant radiotherapy and was being followed a year later, without detectable metastasis. Table 2 describes the demographic, clinical, anatomopathological, staging and outcome data of this group of patients.
Distribution of cases of cutaneous malignant melanoma of the head and neck submitted to sentinel lymph node screening according to demographic, clinical, anatomopathological variables and anatomopathological results related to cervical metastasis.
| Sentinel positive (n = 3) | Sentinel negative (n = 28) | p | |
|---|---|---|---|
| Gendera | |||
| Female | 2 (66.6) | 14 (50.0) | p = 0.05 |
| Male | 1(33.4) | 14 (50.0) | |
| Age (SD)b | 63.33 (9.3) | 62.07 (13.6) | p = 0.87 |
| Primary tumor site | |||
| Malar | 1 (33.4) | 10 35.8) | |
| Nasal | 0 (0) | 3 (10.7) | |
| Ear | 1 (33.3) | 3 (10.7) | NA |
| Scalp | 1 (33.3) | 2 (7.1) | |
| Temporal | 0 (0) | 2(7.1) | |
| Others | 0 (0) | 8 (28.6) | |
| Level of Clark | |||
| II | 0 (0) | 0 (0) | |
| III | 0 (0) | 7 (25.0) | NA |
| IV | 1 (33.3) | 13 (46.4) | |
| V | 2 (66.7) | 8 (28.6) | |
| Breslow index (SD)b | 11.37 (0.5) | 4.37 (5.0) | p = 0.02 |
| Ulcerationc | |||
| Present | 0 (0.0) | 8 (28.6) | |
| Absent | 3 (100.0) | 20 (71.4)) | p = 0.65 |
| Mitotic index (SD)b | 8.0 (3.6) | 4.7 (6.7) | p = 0.42 |
| TNM staging | |||
| T1 | 0 (0) | 4 (14.3) | |
| T2 | 0 (0) | 11 (39.3) | NA |
| T3 | 0 (0) | 5 (17.8) | |
| T4 | 4 (100.0) | 8 (28.6) | |
| Deatha | |||
| Yes | 2 (66.6) | 5 (17.8) | p = 0.12 |
| No | 1 (33.3) | 23 (82.1) |
SD, standard deviation; NA, p value calculation not applicable.
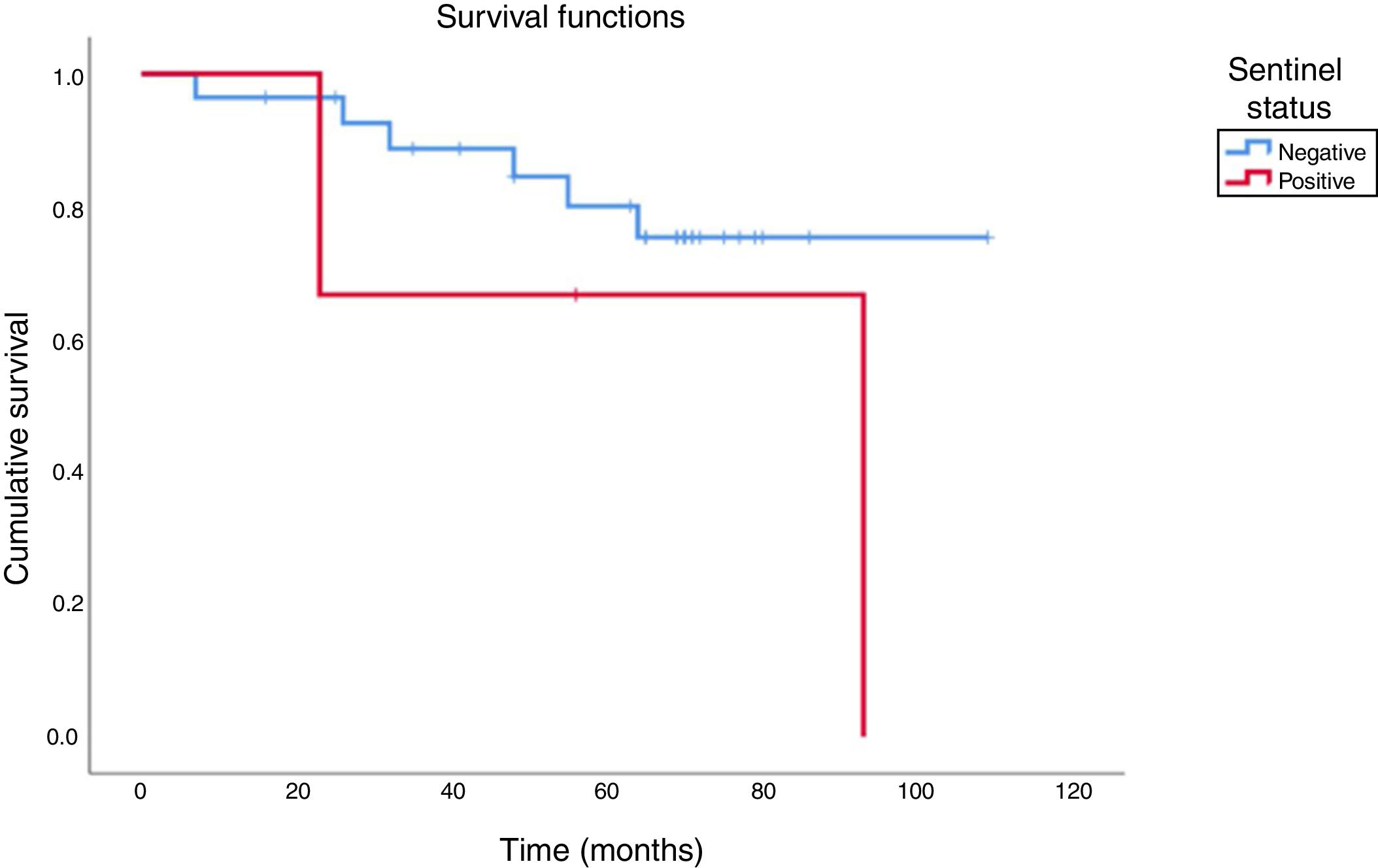
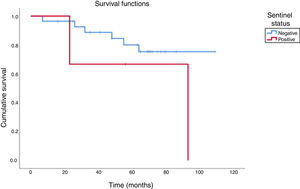
Of five patients with cervical metastases detectable on admission, four underwent modified radical neck dissection and one underwent a classic radical dissection together with a contralateral modified radical dissection. The five patients underwent adjuvant radiotherapy; three died of metastasis (two in lung, one in liver) and two remained alive until the last followup information, with an overall survival of 40%. When we compare these cases to patients with positive sentinel lymph node in the series, a similar pattern was observed: of the three patients with a positive sentinel lymph node, one died of a second primary tumor in the supraglottic region and the other had pulmonary metastasis, resulting in an overall survival of 33.3%. Finally, the survival of patients with a negative sentinel lymph node was 82.2%. Fig. 2 shows the comparison of the cumulative overall survival rate between the groups with positive and negative sentinel lymph nodes.
DiscussionThis series consisted of patients with melanomas showing greater thickness than those found by other authors.12 The mean Breslow index was 3.0 mm in those with negative sentinel lymph nodes and >10.0 mm among positive ones. Stephansson et al., studying the evolution of Breslow thicknesses, appreciates this finding even more, when verifying a decline in the median of Breslow thicknesses, from 2.15 mm (between 1980–1989) to 0.9 mm (between 2000–2009), in a study of 854 cases of cutaneous melanoma recorded in the Icelandic Cancer Registry Database.13
Although the importance of the sentinel lymph node biopsy has grown in light of the indication of adjuvant therapy with immunotherapeutic agents, it is noteworthy that many advanced cases had a negative sentinel lymph nodes in this series. Micro-metastases were found only in cases with a Breslow index >7.0 mm.
The sentinel lymph node positivity, in general, is around 20%.14 In this series, although selected by advanced cases from the public health system, the frequency of cases with positive sentinel lymph nodes was lower than expected, 9.7%, although it is known that the positivity in the head and neck subsites is lower than in other regions.8 It is likely that the heterogeneity of cervicofacial cases, which include subsites as distinct as the scalp and malar regions, can explain in part the differences found.15 However, it is noteworthy that three quarters of the sample consisted of tumors in stages T3 and T4,2 in the group of cases submitted to sentinel lymph node biopsy.
The screening for sentinel lymph nodes in the head and neck region, especially after the introduction of SPECT-CT as a tumor location test, is a procedure with little morbidity and translates into an excellent staging data. With the help of the intraoperative probe and the injection of patent blue, deep tissue dissection is minimal, when compared to elective dissection.
Historically, we have observed technical improvement in the screening for cervical sentinel lymph nodes. In this series, we reported only one false-negative case in 28 (frequency of 3.5%), in which the patient had cervical metastasis 5 years after a negative sentinel lymph node screening. A prospective assisted-fluorescence study followed 125 patients with cutaneous melanoma, 24.8% located in the head and neck region. Two cases of false negatives were found, one of them being an in-transit metastasis, resulting in a frequency of false negatives of 2% and 1% of adjusted frequency, modest values that corroborate the present case series.16
In this study, the malar region was mentioned as the site of 34.7% of primary tumors, totaling 17 patients, of which 11 (64.7%) had tumors with sufficient thickness to undergo sentinel lymph node biopsy. In this group, only 2 (11.7%) died due to recurrence or metastasis, compared to a rate of 34.4% of deaths in patients with primary tumors in other locations. The probable association may be the higher occurrence of the malignant lentigo subtype in this region,15 which is associated with lower aggressiveness. Similarly, when comparing a group of patients with non-malar head and neck melanomas with another in the malar region, Tas and Erturk observed a greater association with the malignant lentigo subtype, in addition to a higher overall survival rate.17
Another important prognostic parameter, the mitotic index, was emphasized. There were no deaths among patients with zero mitotic index and the cumulative survival was markedly different, according to the findings above or below the best discriminatory value. These data had a more relevant prognostic impact than the sentinel lymph node, with the caveat that the low number of positive sentinel lymph nodes must have contributed to this result. The mitotic index also emerged in Balch's analysis as a powerful predictor of survival. Information extracted from the AJCC Melanoma Staging Database demonstrated a strong correlation between the increase in the mitotic index and the decline in survival rates (p < 0.0001).3
The prognostic importance of the sentinel lymph node biopsy has been discussed since its introduction in clinical practice.18,19 Even in this series, in which positive sentinel lymph nodes were rare, it is noteworthy that 66.6% of patients with micro-metastases died as opposed to 17.8% among the negative ones. Likewise, in a study of a series of advanced melanomas, Gajdos et al. found a relative risk of death of 2.28 times for patients with positive sentinel lymph nodes, when compared to those without micro-metastases, as confirmed by the multivariate analysis.20
The mean survival of patients with cervical metastasis at diagnosis was 20.8 months. In those with positive sentinel lymph nodes, this time was 34.2 months. These data are compatible with the case studied by Balch et al., which showed that, at two and a half years of followup, there was a cumulative survival of 50% for patients with metastasis.3
In the present series, patients were treated with surgery until 2014, and cervical dissection allowed locoregional control in a period when adjuvant immunotherapy was not routinely established.
ConclusionIn this series of advanced head and neck melanomas treated by the public health system, SUS, in Brazil, micro-metastases showed a low frequency (9.6%), exclusively in thick melanomas (>7 mm), showing positivity only in advanced cases.
The frequency of false-negative results in the sample was 3.5%, similar to other studies carried out under equivalent technical conditions.
The impact of the mitotic index on patient prognosis was as relevant as the sentinel lymph node status in this study; there were no deaths among patients with zero mitotic index tumors and the cumulative survival rates were markedly different, according to the mitotic index values below or above the best discriminatory value; however, 66.6% of patients with micro-metastases died, against 17.8% among the ones with negative results.
Finally, the study calls attention to the severity of the melanoma diagnosis, and, although positive sentinel lymph nodes are rare, it demonstrates the prognostic importance that sentinel lymph node screening continues to have in the scenario of the appropriate treatment of melanomas in the cervicofacial region. We also call attention to the fact that the existence of more services with integrated nuclear medicine would expand its accessibility, providing patients with adequate disease staging and, consequently, the choice of a more assertive treatment.
Conflicts of interestThe authors declare no conflicts of interest.
Peer Review under the responsibility of Associação Brasileira de Otorrinolaringologia e Cirurgia Cérvico-Facial.