Vascular endothelial growth factor is thought to be an important angiogenic factor involved in tumor growth, progression, and metastasis.
ObjectiveThe present study evaluated the relation between tissue expression, serum and salivary levels of vascular endothelial growth factor in head and neck squamous cell carcinomas, and their correlation with clinicopathologic features.
MethodsSamples were collected from 30 patients with head and neck squamous cell carcinomas and 24 healthy volunteers. Immunohistochemical analysis was used for tissue expression and enzyme-linked immunosorbent assay was employed to measure serum and salivary levels.
ResultsNo vascular endothelial growth factor staining was observed in normal tissues, whereas vascular endothelial growth factor expression was seen in 6 patients (20%). Mean serum level of VEGF was 83.7±104.47 in patients and 50.04±32.94 in controls. Mean salivary level of vascular endothelial growth factor was 174.41±115.07 in patients and 149.58±101.88 in controls. No significant difference was found by Mann–Whitney test between controls and patients (p=0.411, p=0.944, respectively). No correlation was found between vascular endothelial growth factor tissue expression and its serum and salivary level.
ConclusionOverexpression of vascular endothelial growth factor was found in head and neck squamous cell carcinoma patients, suggesting its role in the pathogenesis of head and neck squamous cell carcinoma, but no relation was found between tissue expression, serum levels, and salivary levels of this marker.
Acredita-se que o fator de crescimento vascular endotelial (FCEV) seja um importante fator angiogênico envolvido no crescimento, na progressão e na metástase tumoral.
ObjetivoO presente estudo avalia a relação entre a expressão tecidual e os níveis séricos e salivares do FCEV em carcinomas de células escamosas da cabeça e pescoço (CCECPs) e sua correlação com aspectos clinicopatológicos.
MétodoForam coletadas amostras de 30 pacientes com CCECP e de 24 voluntários saudáveis. Utilizamos análise imuno-histoquímica para a expressão tecidual e ELISA para determinação dos níveis séricos e salivares.
ResultadosNão foi observada coloração para FCEV nos tecidos normais, enquanto que foi observada expressão de FCEV em seis pacientes (20%). O nível sérico médio de FCEV foi 83,7±104,47 em pacientes e 50,04±32,94 em controles. O nível salivar médio de FCEV foi de 174,41±115,07 em pacientes e 149,58±101,88 em controles. Não foi observada diferença significativa pelo teste de Mann-Whitney entre controles e pacientes (respectivamente, p=0,411, p=0,944). Não foi observada relação entre a expressão tecidual de FCEV e seus níveis séricos e salivares.
ConclusãoA expressão elevada de FCEV foi observada em pacientes com CCECP, e isso sugere seu papel na patogênese de CCECP, mas não foi observada relação entre a expressão tecidual e os níveis séricos e salivares desse marcador.
Angiogenesis is an important phenomenon in the development of tumors and metastasis, which is created by secretion of several growth factors by means of tumor cells and tumor surroundings. Although tumor development is a multiple phase process, angiogenesis is necessary for tumor growth and metastasis.1,2 Vascular endothelial growth factor (VEGF) is known to be a fundamental regulator of angiogenesis that accelerates cellular proliferation, vascular permeability, and endothelial cell migration, as well as functioning as an apoptosis inhibitor.3 VEGF is a heparin binding glycoprotein and its gene is located on chromosome 6.1 This protein is the most important of VEGF family and has a role in vascular permeability.4 Inhibition of VEGF activity leads to diminished growth and decreased tumor progression, which suggests that it has an important role in initiation of tumor angiogenesis.5 Furthermore, it has been determined that hypoxia can increase VEGF expression, and that VEGF overexpression leads to angiogenesis in the hypoxic area of tumors.6
VEGF is expressed in several malignant tumors, which indicates its important role in the process of angiogenesis. Over-expression of VEGF has been found in solid tumors, such as those of the colon, kidney, breast, brain, pancreas, bladder, ovary, stomach, lung, and oral cavity.7
Regarding the role of VEGF in the angiogenic process, the aim of this study was to investigate the expression, serum and salivary levels of this marker in patients with head and neck squamous cell carcinoma (HNSCC).
MethodsIn this cross-sectional study, 30 patients with HNSCC (21 males and nine females), who were referred to Khalili and Chamran Hospitals of Shiraz University of Medical Sciences, were examined. The control group consisted of 24 healthy individuals (16 male and eight females) who were matched with the patients group in terms of age and sex.
Those suffering from any systematic disease, active infection, autoimmune disease, inflammatory disease, or periodontal problems were excluded from the study. As for the patients group, those who had a history of radiotherapy, chemotherapy, or other cancers were excluded.
H&E slides were examined in patients with HNSCC and those with sufficient tissue were selected for immunohistochemical (IHC) studies. Clinical information of patients, including age, sex, tumor location, tumor size, metastasis stage and grade, as well as tobacco consumption habits, were collected from the patients’ records. The Ethics Committee of Shiraz University of Medical Sciences approved the study and all patients signed an informed consent.
To prepare serum, 5cm3 of blood was drawn from the patients’ veins, who were NPO for 12h, in the morning before surgery. The blood samples were immediately centrifuged at 3000rpm for 10min. The serum was then separated and stored at −80°C until analysis.8
In order to collect saliva, unstimulated whole saliva samples were collected from the patients, who were NPO for 12h, in the morning before surgery.
The patients were asked to refrain from eating, drinking, and smoking for 30min. Then, the patients’ lips were cleaned and each patient rinsed his/her mouth with water. Approximately 5–10mL saliva was collected from every patient. After centrifugation of the samples (2600×g, 15min, 40°C), they were then stored at −80°C until use.9
Serum and saliva analysisConcentration of VEGF was measured using enzyme-linked immunosorbent assay (ELISA) (Bender Med. Systems GmbH – Germany), according to the manufacturer's instructions.
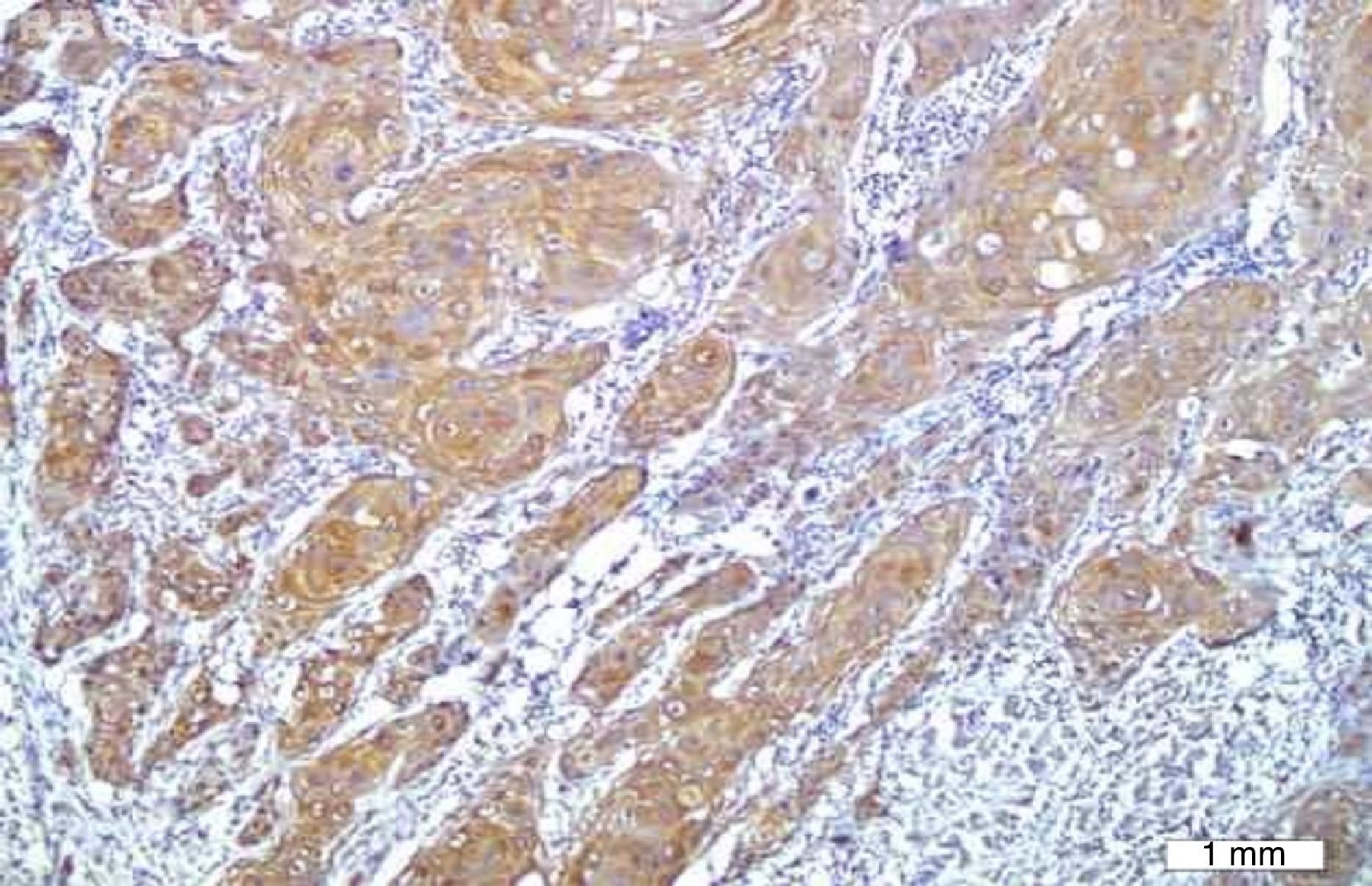
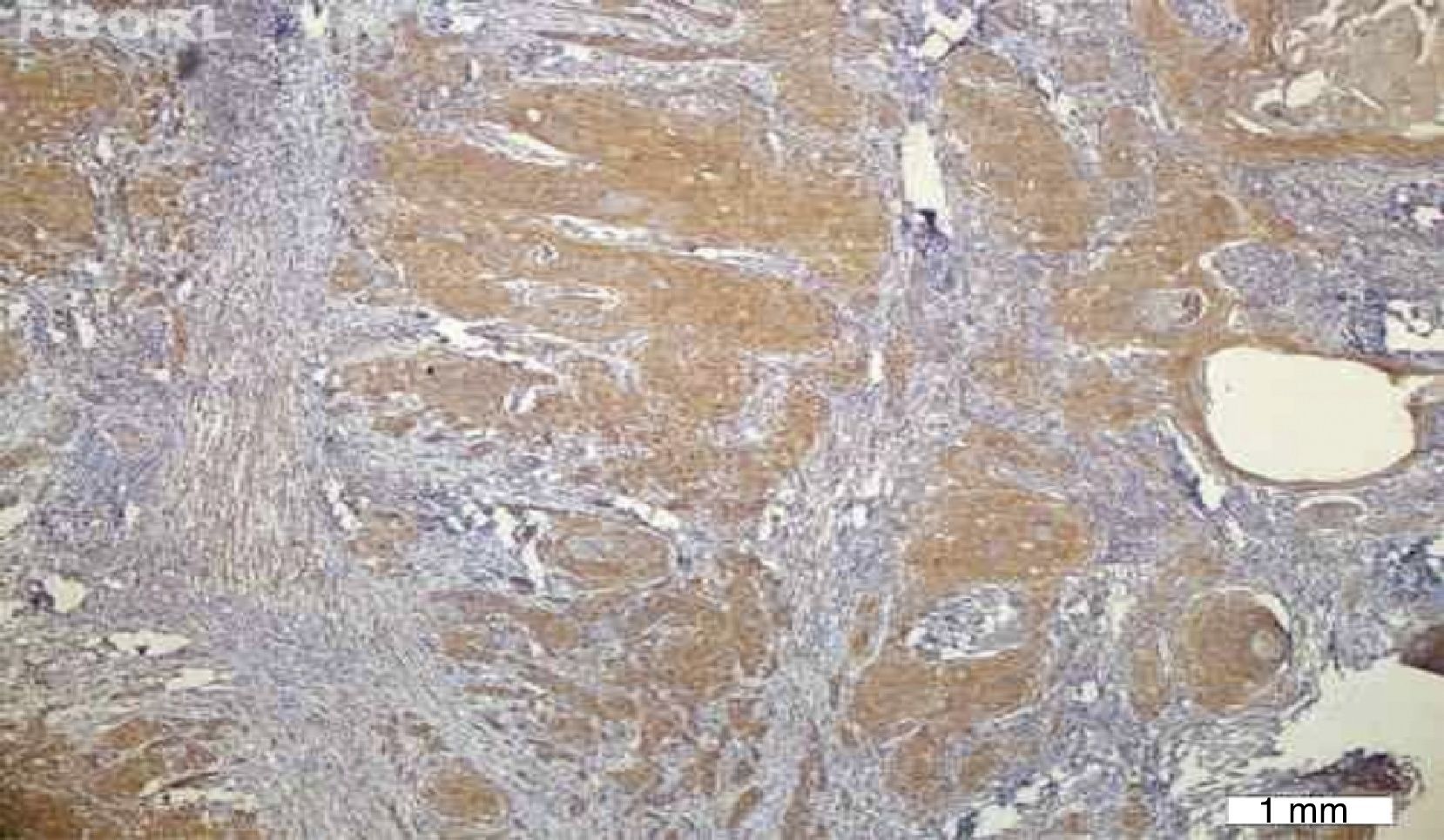
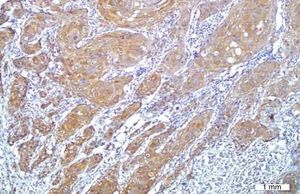
Immunohistochemical stainingIHC staining was performed by using the EnVision® Labeled Peroxidase System (DAKO, Carpentaria, CA, USA). All samples were fixed in 10% buffered formalin and were embedded in paraffin. Sections with 4μm thickness were prepared, deparaffinized in xylene, rehydrated in graded alcohol, and washed with distilled water. Antigen retrieval was performed by using DAKO cytomation target retrieval solution with pH=9, for 20min. Internal peroxidase activity was inhibited by 3% H2O2. Tissue sections were then incubated for 30min with the anti-VEGF antibody (mouse anti-human; DAKO Corporation – Denmark) at a 1:25 dilution. Normal samples were stained with the same amount of antibody used for staining tumor tissues. Omission of the primary antibody was employed as negative control, while pyogenic granuloma was used as positive control. Brown cytoplasmic staining for VEGF was considered as positive.
The stained slides were initially scanned at low magnification. For the slides showing heterogeneous staining, the regions with higher staining were studied. Five fields were randomly chosen, 500cells were counted, and the percentage of staining was calculated. The extent of staining was classified as: 0 if 0–10% of tumor cells were stained, 1 if 11–25% of tumor cells were stained, 2 if 26–50% were stained, and 3 if more than 50% were stained.
Statistical analysisAfter entering the data into the statistical software (SPSS 18.0), the normality of the data was first examined using the Kolmogorov–Smirnov test. As the variables under study were not normally distributed, Mann–Whitney, Kruskal–Wallis, and chi-squared tests were used in order to compare the two groups. Differences were considered significant at p<0.05.
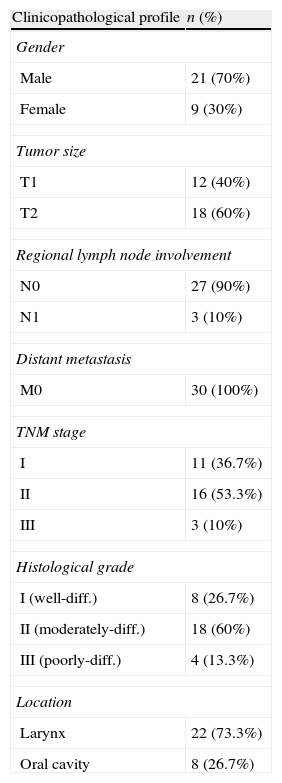
ResultsDescription of variablesThe present study was performed on 30 patients with HNSCC (21 males and nine females) with a mean age of 56.76±12.46 years. Clinicopathologic characteristics of the patients are shown in Table 1.
Clinicopathological profile of 30 head and neck squamous cell carcinoma patients.
| Clinicopathological profile | n (%) |
| Gender | |
| Male | 21 (70%) |
| Female | 9 (30%) |
| Tumor size | |
| T1 | 12 (40%) |
| T2 | 18 (60%) |
| Regional lymph node involvement | |
| N0 | 27 (90%) |
| N1 | 3 (10%) |
| Distant metastasis | |
| M0 | 30 (100%) |
| TNM stage | |
| I | 11 (36.7%) |
| II | 16 (53.3%) |
| III | 3 (10%) |
| Histological grade | |
| I (well-diff.) | 8 (26.7%) |
| II (moderately-diff.) | 18 (60%) |
| III (poorly-diff.) | 4 (13.3%) |
| Location | |
| Larynx | 22 (73.3%) |
| Oral cavity | 8 (26.7%) |
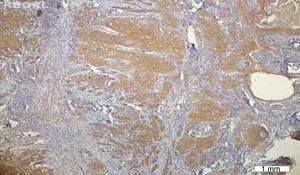
No VEGF staining was observed in normal tissues, whereas VEGF expression was observed in six patients (20%); four had scores of 1 and two had scores of 2. VEGF expression was not seen around keratin pearls (Figs. 1 and 2).
The results indicated that the tissue expression of VEGF between cases and controls showed a significant difference (p: 0.023), such that it was higher in the HNSCC group than the controls. There was no apparent correlation in VEGF expression with the clinicopathological features, such as stage, tumor size, nodal status, and histological grade.
The mean serum level of VEGF was 83.7±104.47 in patients and 50.04±32.94 in controls. The mean salivary level of VEGF was 174.41±115.07 in patients and 149.58±101.88 in controls. No significant difference was found by Mann–Whitney test between controls and patients (p=0.411, p=0.944, respectively).
No significant correlation was found by Mann–Whitney test between VEGF tissue expression and its serum and salivary levels (p=0.517, p=0.716, respectively). The relationships between serum and salivary levels of VEGF and other variables such as age, sex, M, N, T, grade, and stage were separately examined, and no significant relationship was found between serum or salivary levels and the mentioned variables.
DiscussionVEGF has been recognized as an effective factor for induction of angiogenesis and is a potent mitogen for endothelial cells.10 In the present study, the expression of VEGF was seen in 20% of tumor samples, while none of the tissues of control group were positive, and there was significant difference regarding the expression of VEGF. The increased expression of VEGF in the present study is in accordance with the other studies which were performed on oral SCC and HNSCC.10–12 However, the rate of expression of VEGF in the present study was low compared to other studies, which may be due to few patients in this study when compared to other studies. The relationship between the expression of VEGF and the parameters such as tumor differentiation, lymph nodes metastasis, and depth of invasion has been investigated in different studies.13 The results regarding the investigation of relationship between the expression of VEGF and the above-mentioned factors in HNSCC were different. Tse et al.14 found that there was no relationship between VEGF expression and histopathologic grade and location of tumor, but that increased VEGF expression resulted in decreased survival rate. Kyzas et al.15 found no relationship between the expression of VEGF, grade, and stage of head and neck carcinoma. They declared that the expression of VEGF in benign lesions was greater than in carcinomas and dysplasia; in addition, they found that the expression of VEGF has a key role in adjusting the normal physiologic condition of the mucosa. In this study, there was no relationship between the VEGF expression and clinicopathologic factors. Controversies and different findings may be due to few samples, different grading of VEGF expression, variety of tumors with regard to the primary location, and the different stages of the patients between the present study and other studies. In the present study, most of the patients were in stages I and II. In this study, serum level of VEGF in patient group was slightly higher than control group; nonetheless, this difference was not significant (p>0.05). In contrast in the other studies, increased serum level of VEGF was observed in the patient group.7,16 A strong relation was seen between VEGF serum level and HNSCC in patients with advanced stages.17 In fact, in the other studies, the majority of patients were in advanced stages, different from the present study in which the majority of patients were in stages I and II without distant metastasis. Thus, it is more accurate to compare this study with similar studies such as that of Wu and Meyer.18,19 In their study, most of the patients were in stages I and II, and most of the lesions were located in the larynx. No significant elevation in VEGF level was observed in their study. In the current study, there was no relation between the VEGF serum level and clinicopathologic factors, while in the study conducted by Shang et al.,8 elevated level of VEGF was present in patients with lymph node metastasis. However, in a study by Meyer et al.,18 which is quite similar to the present study regarding the selection of samples, the stage, and location (samples of larynx were investigated), there was no relationship between the circulating VEGF level and clinicopathologic factors. Saliva is one of the body fluids which is easily accessible and its preparation is noninvasive. It is known as the “mirror of body”, and its application was investigated in the diagnosis of systemic diseases and tumor markers.20 Saliva is identified as a source of VEGF. It has been demonstrated that physiologic and pathologic angiogenesis in oral mucosa and salivary tissues is regulated by salivary VEGF.21 The presence of VEGF in the saliva of normal patients suggests the important role of VEGF in maintaining the homeostasis of mucous membranes.22 In the present study, the VEGF salivary level in patients with HNSCC was higher than that of the control group, but there was no statistically significant difference. In a study by Upile et al.,23 the salivary level of VEGF165 was examined, and the results demonstrated that the VEGF salivary and serum levels in patients were significantly higher than those of the control group. In their study, the level of VEGF165 was investigated by immunoassay, while in the current study, the salivary levels of various isoforms of VEGF was investigated by ELISA; this difference may result from the diversity in the measurement of isoforms. In the present study, there was no relationship between the salivary level of VEGF and clinicopathologic factors. In the study by Upile et al.,23 the relationship between the VEGF salivary level and clinicopathologic factors was not investigated. In reviewing the article, the present authors did not find any studies which investigated the relationship between the salivary level of VEGF and clinicopathologic factors.
ConclusionIn the present study, overexpression of VEGF was found in HNSCC patients, which suggests its role in the pathogenesis of HNSCC, but no relation was found between the tissue expression, serum level, and salivary level of this marker.
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to thank the Vice-Chancellery for Research of Shiraz University of Medical Sciences for providing financial support for this study (Grant#90-5551). This manuscript is relevant to the post graduate thesis of Dr. Marzieh Hamzavi.
Please cite this article as: Andisheh-Tadbir A, Hamzavi M, Rezvani G, Ashraf MJ, Fattahi MJ, Khademi B, et al. Tissue expression, serum and salivary levels of vascular endothelial growth factor in patients with HNSCC. Braz J Otorhinolaryngol. 2014;80:503–7.