Glomus tumors (GTs) are benign tumors formed at the anastomosis of arteries and veins, affecting blood flow and temperature control. The tumor can occur in any part of the body. Owing to little data concerning tracheal glomus tumors (TGTs) of uncertain malignant potential, more accumulated cases are required to clarify its characteristics. In this report, we also review relevant literature and discuss the common features and treatments of GTs.
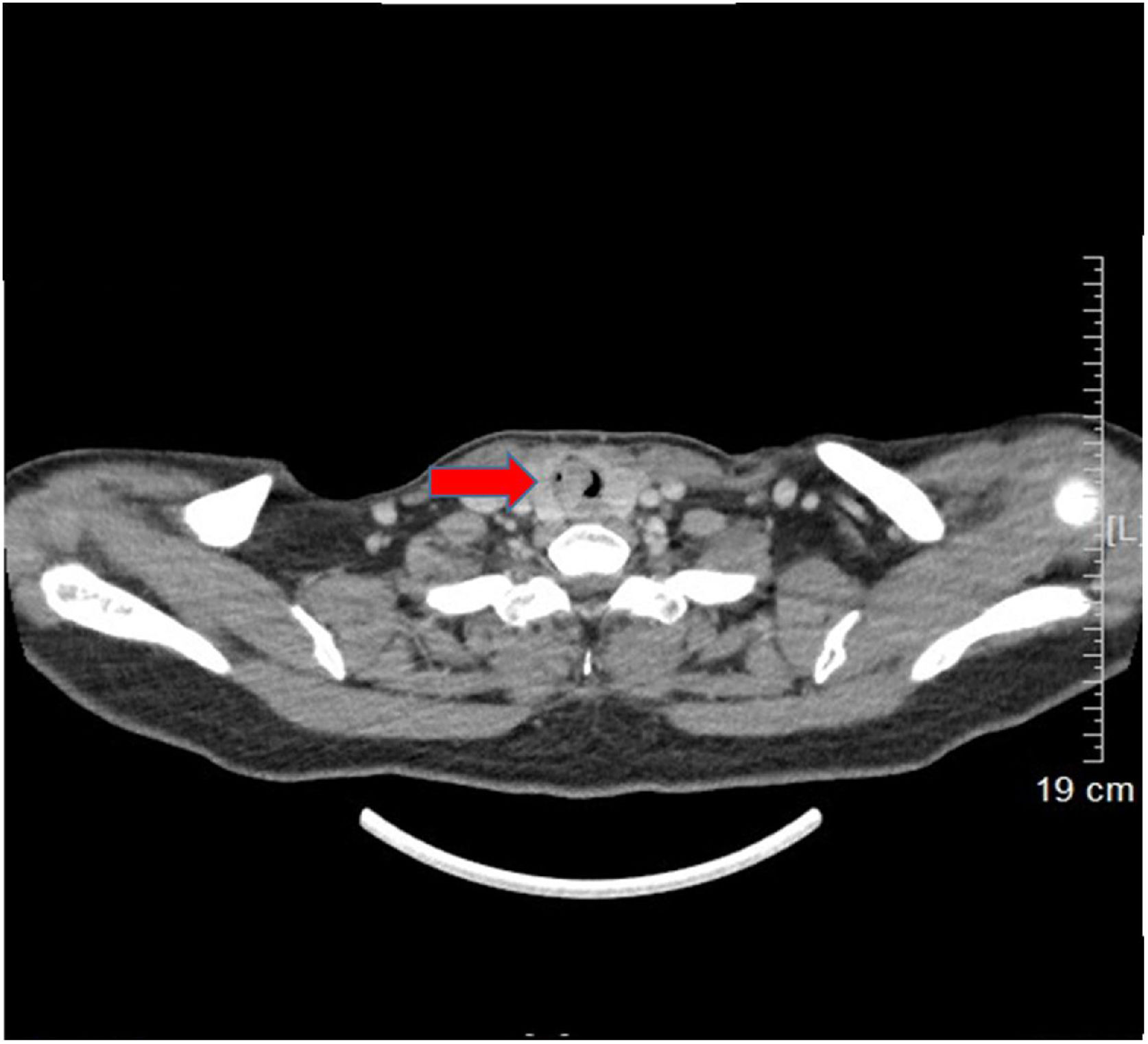
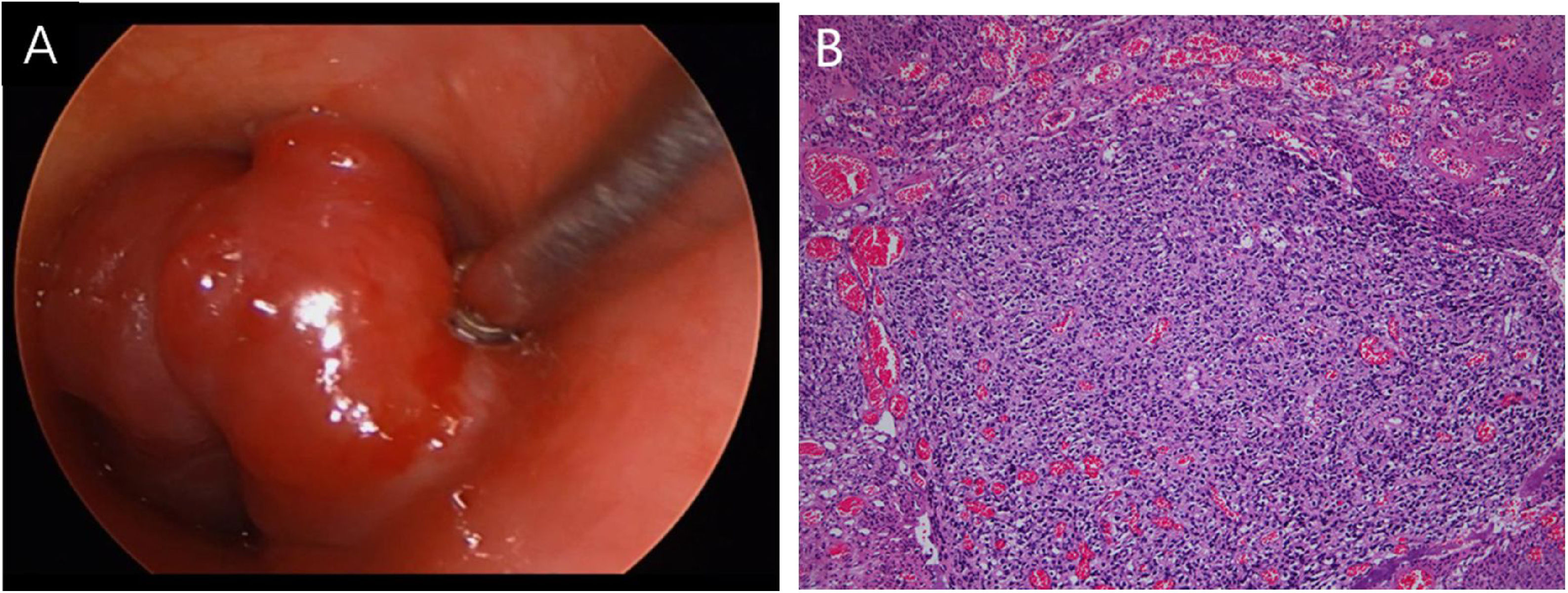
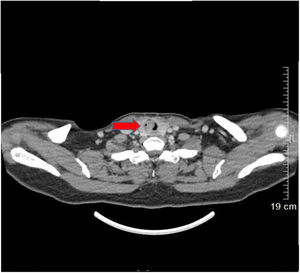
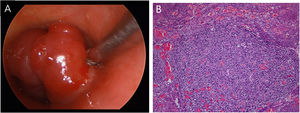
Case reportA 53-year-old female presented with a one-year history of cough and intermittent shortness of breath without hemoptysis or smoking history. She was admitted to the Respiratory Department due to an initial diagnosis of chronic obstructive pulmonary disease (COPD). No improvement in shortness of breath was observed after receiving long-term treatment for spasm and asthma. Spiral computed tomography (CT) with three-dimensional reconstructions in conjunction with tracheal CT enhancement scan showed enhanced tissue in the initial segment of the trachea, measuring approximately 1.4 × 0.9 × 1.4 cm (Fig. 1). A polypoid mass under the glottis almost completely blocked the lumen and moved in tandem with the patient’s breathing. The patient was referred to Otolaryngology-Head and Neck Surgery Department subsequently. To ensure the airway remained unobstructed, we performed a temporary tracheotomy. During the operation, the tumor of the subglottic area, arising from the posterior wall of the trachea was detected (Fig. 2A). The pedicle was resected, and the residual was completely cauterized with the help of Harmonic scalpel.
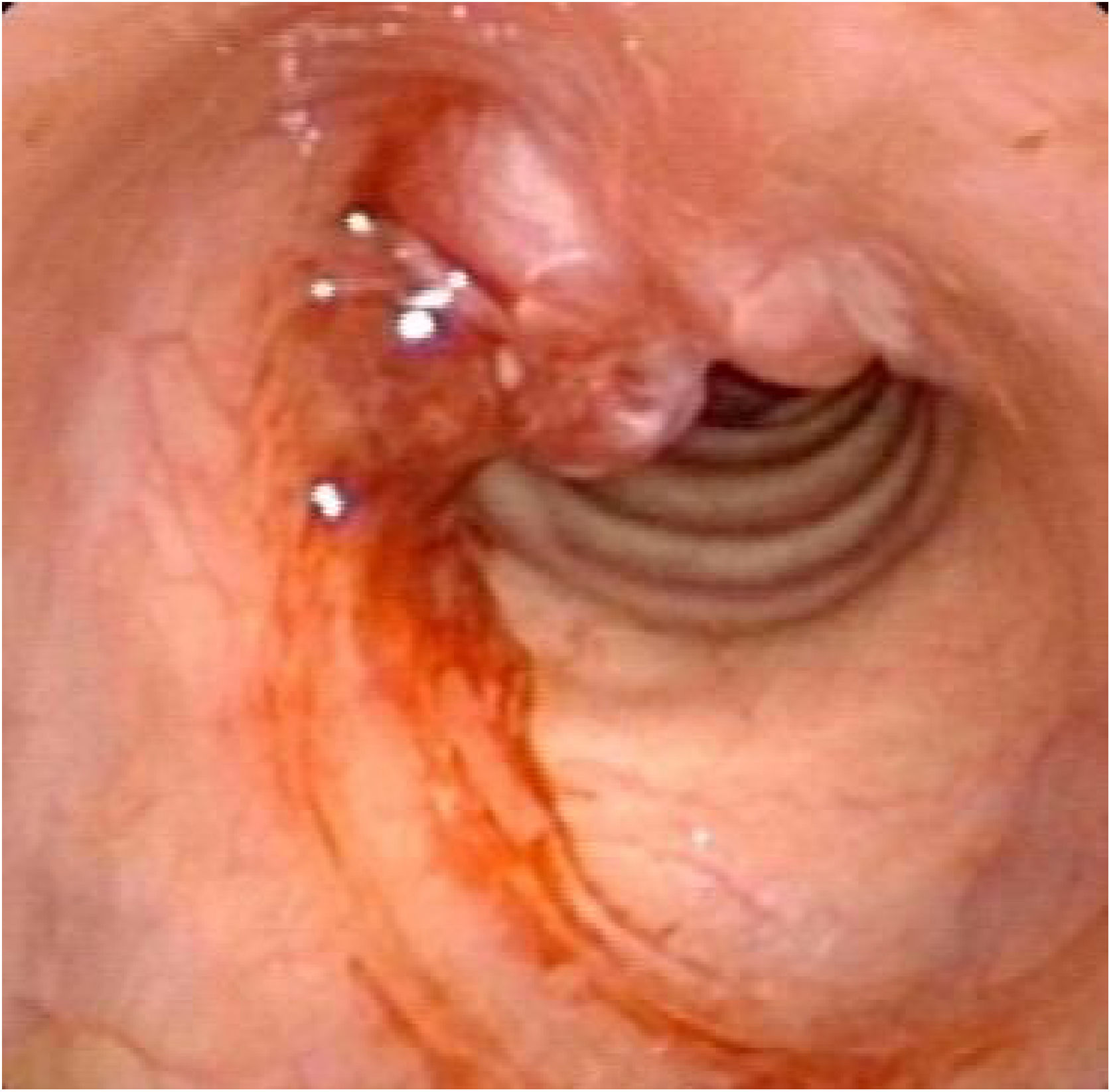
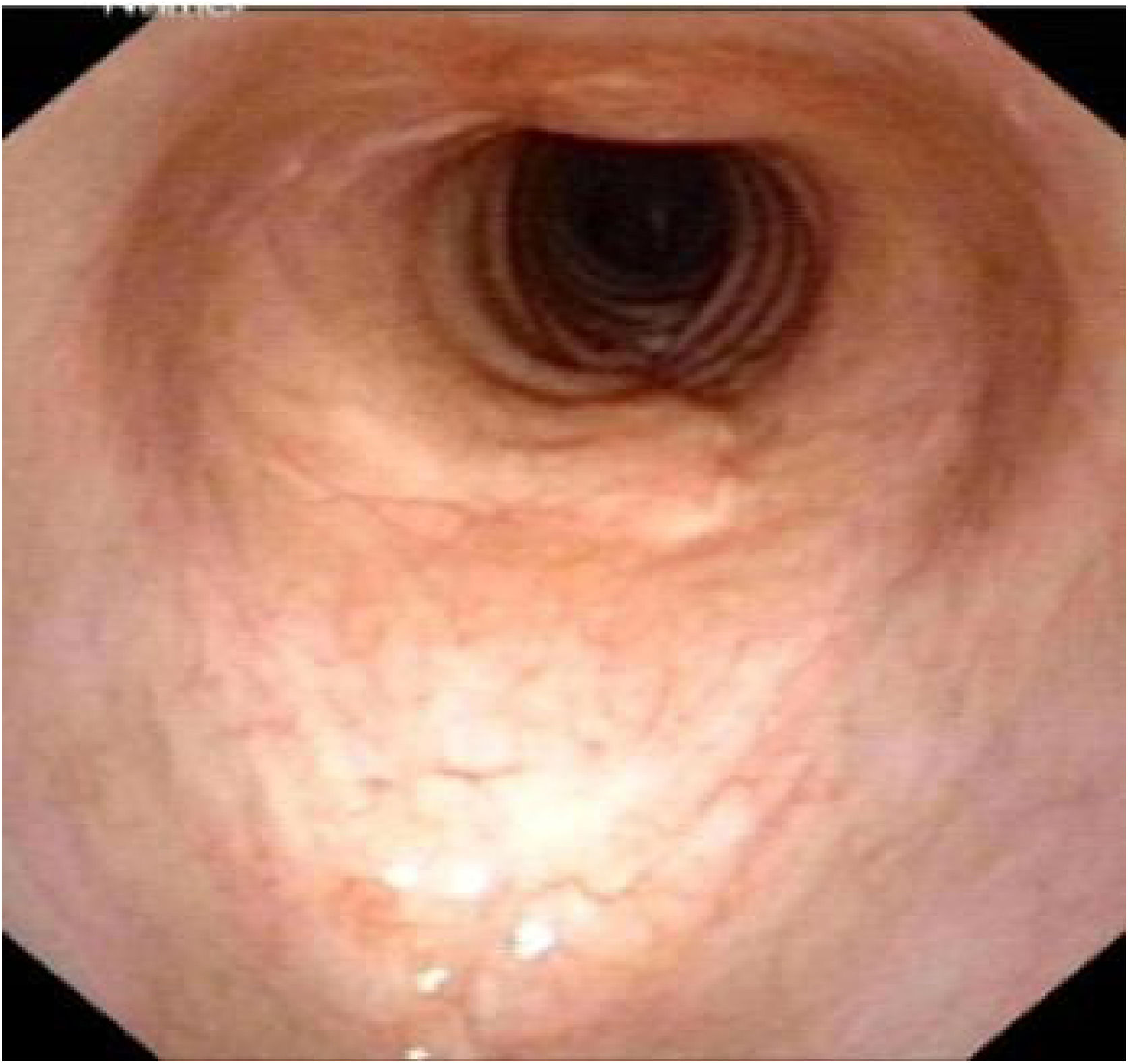
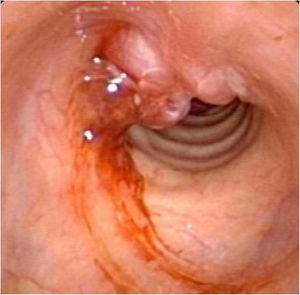
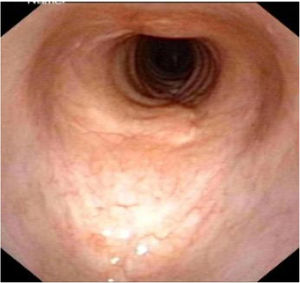
Postoperative pathology (Fig. 2B) revealed focal eosinophilic round cells arranged like hemangiopericytoma around vessels. These cells were positive for vimentin and smooth muscle actin antibody (SMA), synaptophysin (Syn), CD56, S-100, and the Ki67 proliferation index was around 5%. Cells were negative for chromogranin-A (Cg-A), casein kinase (CK), CD34 and epithelial membrane antigen (EMA). Two weeks after the operation, a tracheal CT scan and electronic laryngoscopy were performed, demonstrating healing and scarring. The patient recovered well, and the tracheostomy tube was removed. After one year, hemoptysis occurred, and the tumor was found to have recurred (Fig. 3), expanded upward along the midline of the original tracheotomy position. The tumor and the partial tracheal sleeve resection were subsequently carried out. The patient recovered quickly after the resection and the patient is continually being monitored (Fig. 4). This study was performed under the approval of the ethics committee of our hospital (nº IEC-FOM-013-2.0).
DiscussionGlomus tumors are rare soft tissue neoplasms derived from modified smooth muscle cells.1 GTs were first described by Masson in 1924. The first case was reported in 1950.2 Based on the World Health Organization (WHO) classification of tumors, GTs are commonly classified into three groups including benign GTs, GTs of uncertain malignant potential, and malignant GTs.3 According to the recent WHO classification, the criteria for tumors of uncertain malignant potential is contingent on not fulfilling the criteria for malignancy, and additionally exhibiting at least one atypical feature other than nuclear pleomorphism.4 However, GTs > 2 cm in size with a deep location which were previously diagnosed as malignant are now classified as having uncertain malignant potential.3 In the present case, the tumor was large enough but did not show significant mitotic characteristics or nuclear atypia, thus the diagnosis of uncertain malignant potential.
Clinically, symptoms associated with airway irritation are common in bronchial and tracheal GTs, but asymptomatic GT usually occurs in the peripheral pulmonary parenchyma.5 Depending on the pathology, the main differential diagnoses of tracheal glomus tumor are carcinoid tumor and hemangiopericytoma. CT scan and bronchoscopy are the best diagnostic methods for determining the origin of the tumor currently. However, the complete diagnosis depends on the results of a pathological examination. Due to their abundant vessels, tracheal glomus tumors (TGTs) display an obvious enhanced area in CT images.6 Despite this, it is difficult to distinguish GTs from carcinoid or hemangiomas solely based on radiologic findings as they appear to have a well-circumscribed round mass under contrast enhancement.7 Thus, the identification of the cytological and vascular structural characteristics are particularly important for an accurate classification.
While there is currently no consensus on the treatment of TGTs. Masoum et al.8 reported a case of a 21-year-old patient who underwent GT resection via bronchoscopy, which recurred one year later, followed by subsequent open resection. Jin et al.5 preferred open surgery due to the young age of the patient and the sizable nature of the lesion. In that case, the risk of bleeding and high recurrence was reduced. Recently, Suresh et al.9 introduced a novel treatment for thoracic tracheal GTs, named percutaneous trans-tracheal endoscopic approach (PTEA). The procedure has many obvious advantages in the resection of benign lumen lesions of the lower trachea, it is an easy and better controlled, simple, and less morbid procedure, but further studies are needed to determine the practicability and safety of the method. Hartert et al.10 described a patient who was treated by tracheal sleeve resection via a right posterolateral thoracotomy with end-to-end anastomosis, citing a 96 months followup period without recurrence. Sleeve resection with primary reconstruction of the trachea is the treatment of choice for tracheal glomus tumor.1 So in the second operation, we adopted this approach. The patient recovered well.
To the best of our knowledge, there are 77 published articles regarding GT in the English language medical literature (Table 1), most of the relevant articles being case reports. The most common symptoms reported among symptomatic GT patients are cough 54.55% (42/77), dyspnea 54.55% (42/77) and hemoptysis 44.16% (34/77). Patients with a lower frequency of chest pain (7.9%) were more rarely reported and presented chest tightness, fever, and asthma-like symptoms. Only five patients were reported to be asymptomatic. The locations of TGTs were of superior origin in 20.78% (16/77) of cases, middle in 23.38% of cases (18/77), inferior in 35.06% of cases (27/77), and bronchial in 20.78% of cases (16/77) (Table 2). These distributions suggest that incidences of GT in the lower two-thirds of the trachea are commonplace, perhaps due to the numerous mucous glands and vessels. Most of the published GT cases are benign, with only two cases of uncertain malignant potential previously reported.5 The tumors in these cases were located at the lower third of the trachea or bronchus, both removed by open surgery with no recurrence after two-year follow-up. Except for the location of the bronchial section, a majority of tumors were surgically removed. Only four bronchial GT patients underwent open surgery. Meanwhile, two patients underwent partial or total lung resection. A few patients accepted adjuvant treatments after surgery, including radiochemotherapy, cryotherapy, Nd-YAG laser and argon plasma coagulation (Table 2).
Summary of literature of tracheal glomus tumor.
| First author | Year | Age (years) | Sex | Symptoms | Duration of symptoms before treatment | Tumor site (S/M/I/B) | Size (cm) | Treatment | Follow-up |
|---|---|---|---|---|---|---|---|---|---|
| Hussarek | 1950 | 43 | F | Dyspnea | Not stated | S | Bean-sized | Tracheal resection | Not stated |
| Fabich | 1980 | 63 | M | Cough | 2 years | I | 2.5 × 2.0 × 1.0 | Sleeve resection | Died of complications on the 10th post-op day |
| Warter | 1980 | 69 | M | Dyspnea, hemoptysis | Not stated | M | 2.3 × 1.5 × 1.5 | Segmental resection | Unremarkable |
| Heard | 1982 | 50 | M | Dyspnea | Not stated | I | 2.5 × 1.5 × 1.0 | Sleeve resection | Sepsis, died on the 15th post-op day |
| Ito | 1988 | 51 | M | Hemoptysis | 9 months | S | 1.5 × 1.2 × 1.0 | Segmental resection | 2 years |
| Sheffield | 1988 | 74 | M | Cough, dyspnea | <1 month | I | 2.2 | Endoscopic resection | 7 months |
| Kim | 1989 | 54 | F | Cough, dyspnea, hemoptysis | 3 years (cough) | M | 1.5 × 1.2 | Segmental resection | 13 months |
| Shin | 1990 | 47 | F | Cough, hemoptysis | 3 years | I | 1.5 × 1.0 × 1.0 | Wedge resection | Not stated |
| GarciaPrats | 1991 | 58 | M | Cough, dyspnea, hemoptysis | Several years | M | 2.5 × 1.8 | Segmental resection | 8 months |
| Haraguchi | 1991 | 61 | M | Asymptomatic | Asymptomatic | M | 1.2 | Sleeve resection | Not stated |
| Arapantoni | 1995 | 65 | M | Dyspnea, hemoptysis | 3 months (dyspnea), 3 days (hemoptysis) | I | 4.5 × 3.0 | Endoscopic resection and Nd-YAG | 1 year |
| Koskinen | 1998 | 66 | M | Asymptomatic | Not stated | I | 2.0 × 3.0 | Endoscopic resection, Nd-YAG and external radiotherapy | Not stated |
| Watanabe | 1998 | 43 | M | Hoarseness | Not stated | I | 2.0 × 1.6 × 1.4 | Sleeve resection | 20 months |
| First author | Year | Age (years) | Sex | Symptoms | Duration of symptoms before treatment | Tumor site (S/M/I/B) | Size (cm) | Treatment | Follow-up |
| Menaissy | 2000 | 34 | M | Hemoptysis | 2 months | M | 2.4 × 2.1 × 1.6 | Tracheal resection | 4 months |
| Lange | 2000 | 20 | M | Asthma-like symptoms | < 1 month | B | 1.4 × 1.3 × 0.6 | Bronchial sleeve resection | 9 months |
| Oizumi | 2000 | 48 | M | Hemoptysis | Not stated | B | 0.7 | Bronchial resection | 3 months |
| Gowan | 2001 | 73 | M | Chest pain, dyspnea, hemoptysis | 5 weeks | M | 1.6 × 0.3 × 0.6 | Segmental resection | 6 years |
| Chien | 2003 | 50 | F | Cough, dyspnea, hemoptysis | 8 years (cough and dyspnea), 1 day (hemoptysis) | I | 2.5 × 2.5 × 2.0 | Segmental resection | 1 year |
| Vailati | 2004 | 40 | M | Dyspnea, cough, fever | 6 months | B | 5.0 × 1.5 | Endoscopic resection | 1 month |
| De Weerdt | 2004 | 37 | M | Dyspnea, cough, fever | 2 months | B | Not stated | Endoscopic resection + cryotherapy + Nd-YAG laser | 3 months |
| Nadrous | 2004 | 39 | M | Hemoptysis | 30 months | S | 2.0 × 1.5 × 1.5 | Sleeve resection | 3 months |
| Ren | 2005 | 29 | M | Cough, dyspnea | 2 years (cough), 2 months (dyspnea) | I | 1.7 × 2.0 × 1.7 | Segmental resection | Not stated |
| Takahashi | 2005 | 67 | M | Cough | Not stated | B | 0.8 | Bronchial resection | Not stated |
| Altinok | 2006 | 83 | F | Dyspnea, hemoptysis | 3 months | S | 2.0 × 1.5 × 1.2 | Partial sleeve resection | 1 year |
| Haver | 2008 | 10 | F | Dyspnea, chest pain, cough | 3 weeks | M to I | 1.8 × 1.3 × 1.3 | Tracheal resection | 2 years |
| Colaut | 2008 | 70 | M | Dyspnea, wheezing | 2 months | M | 2.0 × 1.0 × 1.0 | Endoscopic resection and Nd-YAG | 2 years |
| Akata | 2008 | 39 | M | Cough | <1 month | B | 2.5 × 2.5 × 2.0 | Endoscopic resection | 6 years |
| First author | Year | Age (years) | Sex | Symptoms | Duration of symptoms before treatment | Tumor site (S/M/I/B) | Size (cm) | Treatment | Follow-up |
| Shang | 2010 | 59 | M | Chest pain, dyspnea cough | 10 years | I | 2.0 × 1.0 × 0.5 | Endoscopic resection + electrocautery | 1 year |
| Shang | 2010 | 22 | F | Cough, hemoptysis dyspnea | 1 year | I | 1.8 × 1.5 × 1.4 | Endoscopic resection + electrocautery | 1 year |
| Nakajima | 2010 | 30 | M | Hemoptysis | 6 months | B | 1.5 × 1.3 | Bronchial resection | 10 months |
| Parker | 2010 | 43 | F | Dyspnea, chest pain, cough | 6 months | I | 2.0 × 1.6 × 1.5 | Tracheal resection | 11 months |
| Baek | 2011 | 54 | M | Dyspnea, cough | 3 months | M | 1.3 × 1.2 | Tracheal resection | 2 years |
| Mogi | 2011 | 56 | F | Cough, dyspnea | 7 months | I | 1.3 × 1.2 × 1.1 | Tracheal sleeve resection | 9 months |
| Ravenna | 2011 | 79 | F | Dyspnea, cough | 3 months | B | Not stated | Endoscopic resection + Nd-YAG laser | 5 years |
| Sakr | 2011 | 66 | M | Cough, dyspnea | 2 months (cough), 10 days (dyspnea) | S | 1.2 × 0.8 × 2.0 | Endoscopic resection + tracheal sleeve resection | 21 months |
| Okereke | 2011 | 58 | M | Dyspnea | Long term | M | 1.1 | Tracheal resection | 6 months |
| Norder | 2012 | 49 | F | Cough, dyspnea | 3 years | S | 1.2 × 1.1 × 1.1 | Endoscopic resection | Not stated |
| Lange Lazdunki | 2012 | 62 | F | Cough, dyspnea | Not stated | I | 1.6 | Left upper lung resection | Not stated |
| Cukurova | 2012 | 50 | M | Cough, dyspnea, hemoptysis | Not stated | S | Not stated | Endoscopic resection | 3 years |
| Ariizumi | 2012 | 43 | F | Asymptomatic | 3 months | B | Not stated | Tracheal resection | 6 months |
| First author | Year | Age (years) | Sex | Symptoms | Duration of symptoms before treatment | Tumor site (S/M/I/B) | Size (cm) | Treatment | Follow-up |
| Zhu | 2013 | 30 | F | Dyspnea, hemoptysis | 1 year | B | 4.0 × 0.5 × 0.5 | Tracheal resection | 18 days |
| Fan | 2013 | 15 | M | Cough, dyspnea, hemoptysis | 3 months | M | 2.0 × 2.5 | Tracheal resection | 1 year |
| Ghigna | 2013 | 70 | M | Hemoptysis | Not stated | I | 1.6 | Tracheal resection | Not stated |
| Ghigna | 2013 | 40 | M | Hemoptysis | Not stated | I | 1.0 | Tracheal resection | Not stated |
| Chang | 2013 | 76 | M | Fever | 1 week | M | Not stated | Endoscopic resection | Not stated |
| Singh | 2013 | 65 | F | Cough | 3 months | B | 1.2 × 0.4 × 0.5 | Endoscopic resection | Not stated |
| Wei | 2013 | 39 | M | Cough, hemoptysis | 1 year | S | 1.9 × 1.4 × 0.8 | Tracheal resection | 26 months |
| Wei | 2013 | 43 | M | Dyspnea | 3 years | I | 2.0 × 1.5 | Tracheal resection | 19 months |
| Choi | 2014 | 52 | F | Asymptomatic | Asymptomatic | B | 1.6 | Resection of carina and both main bronchi | 3 months |
| Choi | 2014 | 64 | M | Asymptomatic | Asymptomatic | M | 2.6 | Tracheal resection | 2 years |
| Xiong | 2014 | 48 | F | Cough, dyspnea | 6 years | I | 1.2 × 1.0 × 0.8 | Bronchoscopic cryoablation and argon plasma coagulation (APC) | 6 months |
| Xiong | 2014 | 55 | M | Cough, dyspnea; chest pain | 13 days (Hemoptysis); 5 months (Cough and chest pain) | I | 0.5 × 0.3 × 0.3 | Bronchoscopic cryoablation and argon plasma coagulation (APC) | 6 months |
| Wu | 2014 | 58 | F | Hemoptysis | Not stated | I | 2.2 × 2.2 | Tangential resection with spiral tracheoplasty | 2 years |
| Zhang | 2014 | 54 | M | Cough, hemoptysis | 4 years | B | 2.5 × 1.5 × 1.0 | Right total lung resection | 6 months |
| First author | Year | Age (years) | Sex | Symptoms | Duration of symptoms before treatment | Tumor site (S/M/I/B) | Size (cm) | Treatment | Follow-up |
| Zhang | 2014 | 48 | M | Cough | 1 year | B | Not stated | Right upper lung lesion resection | 7 months |
| Huang | 2015 | 39 | F | Dyspnea | More than 1 year | S | 2.5 × 1.2 | Segmental resection | 1 month |
| Liu | 2015 | 39 | F | Dyspnea | More than 1 year | S | 2.5 × 1.2 | Segmental resection | 1 month |
| Li | 2015 | 15 | M | Cough, hemoptysis | 2 months | M | 1.2 × 1.0 × 1.0 | High-frequency electrocautery and APC | 3 months |
| Tan | 2015 | 44 | M | Cough, dyspnea, hemoptysis | 2 months | I | 3.0 × 2.5 × 1.0 | Tracheal resection | 20 months |
| Masoum | 2015 | 21 | M | Cough, hemoptysis | Several months | S | Not stated | Endoscopic resection + tracheal resection | 1 year |
| Fernandez-Bu | 2015 | 48 | M | Hemoptysis and cough | 3 months | I | 2.0 × 2.0 | Endoscopic resection | 2 years |
| Brzezinski | 2015 | 38 | M | Dyspnea | 1 year | S | 1.6 × 1.8 × 0.8 | Tracheal resection | Not stated |
| Rashid | 2015 | 52 | M | Hemoptysis | 3 months | B | Not stated | Endoscopic resection | 6 months |
| Xiong | 2016 | 52 | F | Dyspnea, cough | 6 months | S | 2.0 × 1.0 × 1.0 | High-frequency electrocautery and APC | 9 months |
| Aryan | 2016 | 50 | F | Hemoptysis, cough, dyspnea | 1 week | B | Not stated | Endoscopic resection | Not stated |
| Wang | 2016 | 63 | M | Hemoptysis | 1 week | I | 0.5 × 0.3 | High-frequency electrocautery and APC | 15 months |
| Wang | 2016 | 44 | M | Hemoptysis, cough | 1 week | M | 1.0 × 1.5 | Endoscopic resection + tracheal resection | 8 months |
| Venegas | 2017 | 51 | F | Dysphagia, hemoptysis dyspnea | Several weeks | I | 2.6 × 2.3 × 1.7 | Tracheal resection; | 12 months |
| First author | Year | Age (years) | Sex | Symptoms | Duration of symptoms before treatment | Tumor site (S/M/I/B) | Size (cm) | Treatment | Follow-up |
| Huang | 2017 | 38 | M | Cough, hematemesis | 20 days | M | 2.4 × 2.2 × 2.7 | The video-assisted transthoracic surgery (VATS) | 3 months |
| Suresh | 2018 | 43 | M | Dyspnea | Not stated | M | Not stated | Percutaneous trans-tracheal endoscopic approach (PTEA) | Not stated |
| Gou | 2018 | 30 | M | Cough, expectoration | 1 month | S | 2.0 | High frequency electroexcision | 1 year |
| Gou | 2018 | 47 | M | Hemoptysis | 3 years | I | 1.8 | Tracheal resection, and anastomosis | 1 year |
| Jin | 2019 | 51 | M | Cough | 4 months | I | 2.0 | Segmental tracheal resection | 2 years |
| Hartert | 2019 | 66 | M | Cough, hemoptysis dyspnea | 3 months | I | 1.1 × 2.2 | Thoracotomy with end-to-end anastomosis | 96 months |
| Shao | 2020 | 41 | M | Chest tightness; chest pain; hemoptysis | 1 month (chest tightness; chest pain); hemoptysis (2 weeks) | s | 1.0 × 1.2 × 1.2 | Endoscopic resection | Not stated |
| Present case | 2020 | 53 | M | Cough, dyspnea | 1 year | S | 1.4 × 1.4 × 0.9 | Harmonic scalpel with Video endoscope | 11 months |
We suggest that as respiratory symptoms cannot be effectively resolved, it may be necessary to perfect examinations for rare diseases conscientiously, especially GTs can be easily missed and misdiagnosed. Although there is no cure in this case, initial bronchoscopy intervention plays a key role in timely and effectively restoring the airway of patients with severe symptoms and providing preoperative diagnostic information. The bronchoscopy biopsy should be avoided due to the tumor’s rich vasculature. Complete resection of the tumor remains the basic procedure of treatment. Long-term followup of tracheal conditions after surgery is certainly necessary.
ConclusionTracheal GTs of uncertain malignant potential, while uncommon, currently have no uniform standards for the surgical treatment of GTs and can be easily mistaken for a pulmonary disease if the symptoms are atypical, we should pay attention to it. Radical resection is still worthy of consideration because of the possibility of recurrence in the clinic. Additionally, tracheal stenosis is the most likely complication of concern.
Conflicts of interestThe authors declare no conflicts of interest.
Peer Review under the responsibility of Associação Brasileira de Otorrinolaringologia e Cirurgia Cérvico-Facial.