Aspirin-exacerbated respiratory disease (AERD) consists of a classic tetrad: moderate/severe asthma, chronic rhinosinusitis, nasal polyps, and intolerance to aspirin or other nonsteroidal anti-inflammatory drugs. Clinical control with drugs, surgery, and desensitization are treatment options.
ObjectiveTo evaluate the efficacy and tolerability of aspirin desensitization in patients with AERD.
MethodsPeriodic symptom assessment and endoscopy in patients with AERD undergoing surgery who were desensitized.
ResultsSeventeen patients were desensitized. Eight patients completed the desensitization and were followed for a minimum of a one-year period (mean 3.1 years). These patients showed improvement in all symptoms. Moreover, surgical reassessment was not indicated in any of these patients and there was a decrease in costs with medication and procedures. Eight patients did not complete desensitization, mainly due to procedure intolerance and uncontrolled asthma, whereas another patient was lost to follow-up.
ConclusionAspirin desensitization, when tolerated, was effective in patients with AERD and with poor clinical/surgical response.
A doença respiratória exacerbada por aspirina é composta pela tétrade clássica: asma moderada/grave, rinossinusite crônica, pólipos nasais e intolerância à aspirina ou outro anti-inflamatório não esteroide. Controle clínico com medicamentos, cirurgias e dessensibilização são opções de tratamento.
ObjetivoAvaliar a eficácia e tolerabilidade da dessensibilização à aspirina em pacientes com doença exacerbada por aspirina.
MétodoAvaliação periódica dos sintomas e exame endoscópico em pacientes com doença respiratória exacerbada por aspirina submetidos à cirurgia e dessensibilizados.
ResultadosDezessete pacientes foram dessensibilizados, dos quais oito pacientes completaram a dessensibilização e foram acompanhados pelo tempo mínimo de 1 ano (média de 3,1 anos). Todos referiram melhora de todos os sintomas; não houve nenhuma indicação de reabordagem cirúrgica, e houve redução de gastos com medicações e procedimentos. Outros oito pacientes não completaram a dessensibilização, principalmente por intolerância ao procedimento e descontrole da asma, enquanto outro paciente perdeu o seguimento.
ConclusãoA dessensibilização à aspirina, quando tolerada, mostrou-se eficaz nos pacientes com doença respiratória exacerbada por aspirina com resposta clínica/cirúrgica insatisfatória.
Aspirin-exacerbated respiratory disease (AERD), also described in the literature as Samter's triad and aspirin-induced asthma (AIA), is a clinical syndrome whose symptoms are induced by a non-allergic hypersensitivity reaction, independent of IgE,1–5 to aspirin and/or other non-steroidal anti-inflammatory drugs (NSAIDs), cyclooxygenase-1 (COX-1) enzyme inhibitors. It was originally described by Widal et al.6 in 1922 and by Samter and Beer7 in 1967. The classical presentation comprises the tetrad: moderate to severe asthma, chronic hypertrophic eosinophilic rhinosinusitis, sinonasal polyps, and intolerance to aspirin or other NSAIDs.1
Symptom onset usually occurs in adulthood, usually before the age of 40,1,3 and the number of affected women is higher than that of men.1,2 There is no described association with ethnicity and family history is rarely present.3 Studies show that its prevalence in the general population is 0.3%–0.9%, reaching 10%–20% in patients with asthma, affecting 30%–40% of asthmatics with nasal polyposis and chronic rhinosinusitis.1,3
The physiopathology of AERD is not fully known. The first theory, proposed by Szczeklik in 1988, associated a viral respiratory infection as a trigger for AERD.4 More recent studies have shown the release of cytokines in vitro by lymphocytes infected with respiratory syncytial virus, parainfluenza virus, and rhinovirus. Cytokines recruit, stimulate, and activate inflammatory cells.4,5
Another factor appears to be the increased expression of specific cytokines associated with activation and survival of eosinophils in nasal polyps, such as interleukin 5 (IL-5), GM-CSF (granulocyte-macrophage colony-stimulating factor), and eotaxin, which would increase the intensity of local eosinophilic inflammation.
It is believed that patients with AERD have genetic polymorphisms that lead to reduced activity of the COX-1 isoenzyme and increased affinity of leukotriene receptors, with low production of PGE2 (prostaglandin E2) and low COX-2 expression in nasal polyps. Since PGE2 has anti-inflammatory activity, the inhibition of eosinophil chemotaxis and its activation, and a decreased production of this prostaglandin (PG) would contribute to the development of more severe eosinophilic inflammation. These alterations in the arachidonic acid metabolism lead to an imbalance in the PG/leukotriene ratio in these patients, causing inflammatory alterations in the upper and lower airways.8–11
The ingestion of aspirin (acetylsalicylic acid) or NSAIDs by a sensitive patient inhibits COX-1 and results in the exacerbation of the inflammation already present in the upper and lower airways, with a wide spectrum of severity and manifestations that range from conjunctivitis and rhinitis to laryngospasm and bronchospasm.1 Initially, the clinical manifestation of AERD is nasal congestion, which may be reported by the patient as an upper-airway viral infection that never resolved. Hyposmia or anosmia occurs in most patients with AERD.3 This rhinitis develops into chronic hypertrophic eosinophilic pansinusitis and emergence of nasal polyps, which recur even after surgical excision. Asthma may already be present from childhood or young adulthood, or occur after three months to five years of symptom onset, and is usually moderate to severe. Hypersensitivity skin tests are usually negative in patients with AERD, indicating higher prevalence in non-atopic individuals.12
History of asthma triggered by ingestion of aspirin or other NSAIDs is suggestive of AERD. The challenge test is the gold standard for the diagnosis.1–5,11 The oral route is the most used, because it is more sensitive and its specificity is similar to that of the nasal, bronchial, and intravenous routes.
The management of patients diagnosed with AERD includes nasal surgeries, ASA desensitization, and recommendations on how to completely avoid the use of non-selective COX inhibitors. For those making this choice, it is necessary to have full knowledge of all the drugs that inhibit this pathway, including those with cross-reactivity. It is noteworthy that selective inhibitors of COX-2 can be used in patients with AERD; however, due to the albeit remote possibility of cross-reactions, it is recommended that the first dose of these drugs be administered in a private clinic or hospital.1
However, even if they avoid the use of COX-1 inhibitors, patients with AERD usually have progressive worsening of respiratory disease, even with aggressive surgical treatment and topical and/or systemic corticosteroids and anti-leukotrienes.2,13–15 It is also noteworthy that avoiding the use of aspirin is not always possible, as in cardiovascular disease management. The chance of recurrence of nasal polyposis and the need for new endoscopic paranasal sinus surgery (EPSS) is high in patients with AERD, compared with patients with nasal polyposis who are tolerant to aspirin.16
Currently, there is no biomarker that can predict the disease and airway remodeling activity. Recently, there have been studies that identified serum periostin increases as a useful biomarker for the assessment of airflow limitation in asthmatic patients, indicating Th2 inflammatory response.17,18
The aim of the study was to evaluate the effectiveness of ASA desensitization in patients with AERD treated at the Rhinosinusology Clinic, as well as their tolerance to the procedure.
MethodsThe study was approved by the Research Ethics Committee, Process No. 13091313.7.0000.5440. Patients who had chronic rhinosinusitis with nasal polyps (CRSwNP), moderate/severe asthma, and history compatible with hypersensitivity to aspirin were evaluated from 2008 to 2013. The patients remained moderately symptomatic even if submitted to short pulses of systemic corticosteroids, topical corticosteroid therapy (both applications in each nostril twice a day), and antileukotriene once a day. All underwent oral challenge test with ASA, according to the specific service protocol, establishing the diagnosis of AERD. Of these, 16 accepted the ASA desensitization protocol, which was conducted at the Hospital Allergy and Immunology Clinic. Four weeks after EPSS, patients were hospitalized, properly advised on the need for the procedure, and signed the informed consent.
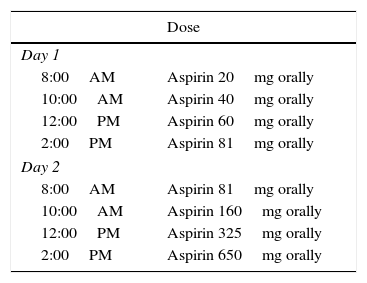
Desensitization protocolInitially, venous access was performed through a peripheral vein. The following medications were available at the bedside for any systemic reaction: methylprednisolone 125mg IV, salbutamol nebulizer (10–20 gts), ranitidine 50mg IV or cimetidine 300mg IV, adrenaline 1:1000 0.3mL IM, and promethazine 50mg (1amp) IV. The pre-medications were prednisone 20mg every 12h and montelukast (Singulair™) 10mg every 12h, 48h prior to desensitization, which were maintained during hospitalization. The use of beta-blockers or ACE inhibitors was not allowed. Procedures were also performed for respiratory/cardiovascular assessment:
- a.
Vital signs every hour during desensitization and, whenever necessary;
- b.
Basal FEV1 through spirometry. A 20% reduction in FEV1 was pre-calculated by multiplying FEV1 in liters by 0.8;
- c.
Previous spirometry (FEV1) at each dose of aspirin, at each hour and when necessary. If the patient had severe dyspnea or decrease in FEV1>20%, salbutamol nebulization was performed;
- d.
If SBP<90mmHg, adrenaline 1:1000 0.3mL was administered IM;
- e.
Rhinorrhea, nasal congestion, and flushing: FEV1 and blood pressure (BP) were checked and verified, if rhinorrhea was accompanied by a decrease in FEV1>20% or systolic BP<90mmHg, and protocol was followed as previously described. For rhinorrhea, nasal congestion, or flushing not accompanied by the abovementioned symptoms, promethazine 50mg IV was administered.
The patient was observed for 3h after the final dose, and then was discharged with the following medications:
- 1.
Aspirin 650mg every 12h, orally;
- 2.
Proton pump inhibitor or H2 blocker was considered for gastric protection;
- 3.
Regularly used medications.
The daily dose of 1300mg was maintained for six months, and after that, the dose was decreased according to clinical parameters (mean of 375–975mg/day). Because the desensitized state is maintained for only two to five days after aspirin use cessation, when the use is below 325mg/day for more than 48h, intake should not be resumed, due to the risk of severe reactions.
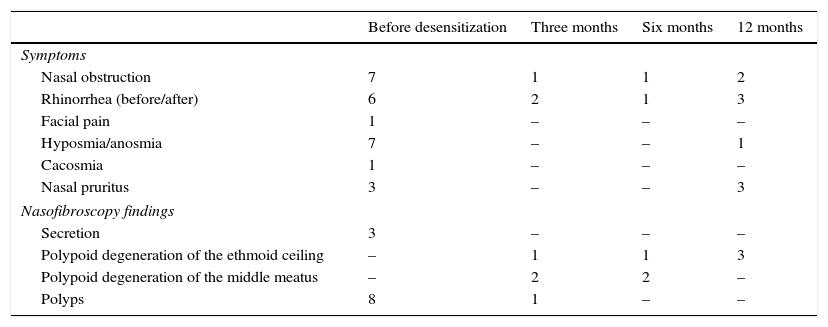
Patients maintained the previously used drugs and were told to come for periodic assessments at the Outpatient Clinic of Rhinosinusitis every three months in the first semester, and every six months thereafter. At these return consultations, the patients were evaluated for the presence of sinonasal symptoms (nasal obstruction, anterior or posterior rhinorrhea, facial pain, hyposmia, cacosmia, and other sinonasal symptoms), and the nasofibroscopy findings (presence of polypoid degeneration in the ethmoid roof, middle meatus, polyps, secretion). The mean interval between recurrence requiring surgery before and after desensitization, time until the diagnosis of AERD and the results of immediate hypersensitivity skin tests, performed to assess the association with atopy, were also evaluated.
A group of eight patients undergoing desensitization did not complete the procedure, or did not persist with the later use of the drug. Patients who refused to undergo desensitization or were lost to follow-up, or who were still undergoing follow-up for a period less than a year after desensitization were not included in this study.
ResultsPatients with CRSwNP and moderate/severe asthma submitted to EPSS were assessed. Among all diagnosed with AERD, seventeen of them, who had already been submitted to at least one surgery, accepted and underwent the desensitization protocol. One patient was lost to follow-up and was excluded from the study. Of the 16 remaining patients, eight completed the procedure, while eight others had the treatment interrupted.
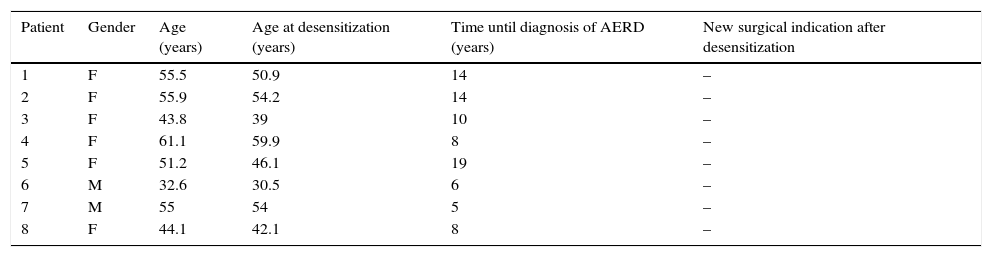
Of the eight patients who completed one year of desensitization, there were two males and six females. The mean time from symptom onset and AERD diagnosis, as well as patients’ ages at the start of desensitization and time of follow-up are described in Table 1. The mean follow-up was 3.1 years after desensitization (ranging between one and five years). Symptom assessment and nasofibroscopy findings before and after desensitization are shown in Table 2.
Patients who completed ASA desensitization.
| Patient | Gender | Age (years) | Age at desensitization (years) | Time until diagnosis of AERD (years) | New surgical indication after desensitization |
|---|---|---|---|---|---|
| 1 | F | 55.5 | 50.9 | 14 | – |
| 2 | F | 55.9 | 54.2 | 14 | – |
| 3 | F | 43.8 | 39 | 10 | – |
| 4 | F | 61.1 | 59.9 | 8 | – |
| 5 | F | 51.2 | 46.1 | 19 | – |
| 6 | M | 32.6 | 30.5 | 6 | – |
| 7 | M | 55 | 54 | 5 | – |
| 8 | F | 44.1 | 42.1 | 8 | – |
F, female; M, male.
Symptoms and nasofibroscopy findings in eight patients undergoing aspirin desensitization during follow-up.
| Before desensitization | Three months | Six months | 12 months | |
|---|---|---|---|---|
| Symptoms | ||||
| Nasal obstruction | 7 | 1 | 1 | 2 |
| Rhinorrhea (before/after) | 6 | 2 | 1 | 3 |
| Facial pain | 1 | – | – | – |
| Hyposmia/anosmia | 7 | – | – | 1 |
| Cacosmia | 1 | – | – | – |
| Nasal pruritus | 3 | – | – | 3 |
| Nasofibroscopy findings | ||||
| Secretion | 3 | – | – | – |
| Polypoid degeneration of the ethmoid ceiling | – | 1 | 1 | 3 |
| Polypoid degeneration of the middle meatus | – | 2 | 2 | – |
| Polyps | 8 | 1 | – | – |
None of the patients followed after desensitization had a new surgical indication. Eight did not complete or did not persist with desensitization. There was a predominance of women (six women and two men), with a mean age of 50 years, 9 months. The mean number of surgeries was 1.75 (range from one to four procedures) prior to desensitization. Three patients did not complete desensitization due to adverse reactions and decompensated asthma. Three others discontinued the use after less than one month of aspirin in full dose due to asthma worsening. There was also a patient who discontinued use due to suspected dengue fever and another abandoned treatment due to fear of a new surgery.
DiscussionAspirin desensitization is an alternative that has shown to be effective in the management of patients with AERD. Studies have shown that the desensitization results in significant improvement of all AERD symptoms, reduction of rhinosinusitis, better control, and reduced doses of corticosteroids used to treat asthma, anosmia/hyposmia improvement,19 decreased need for revision sinonasal surgery, improved tomographic image and quality of life of patients, as well as reduced costs, both regarding procedures and medications.19,20
Long-term treatment with aspirin causes downregulation of IL-4 and subsequent downregulation of the cysteinyl-leukotriene receptors in lymphocytes, which, ultimately, lead to a decrease in the Th2 inflammatory response. Another important aspect of this therapy is the decrease in MMP-9 (matrix metalloproteinase 9), which is important in the airway remodeling process.19 Desensitization can be considered the first choice of treatment for patients with AERD and recalcitrant nasal polyposis, need for repeated sinus surgery, need for systemic corticosteroids, or those with any medical condition requiring chronic aspirin use.21
The recurrence of nasal polyposis after surgery is almost three-fold higher in patients with AERD, when compared to those without it.22 Other studies have shown that desensitization increased the mean length of surgical reintervention from three to approximately six years,23,24 with significant improvement in quality of life, olfactory function, decreased nasal polyps, and asthma control.24 In the present study, all eight patients that maintained desensitization for at least one year showed significant improvement of clinical parameters and nasofibroscopy findings. After a mean follow-up of 3.1 years, no patient was submitted to a new surgical approach.
Desensitization is not risk-free and requires several precautions due to possible complications. Even when conducted in a hospital under stringent medical care and frequent outpatient consultations, many patients do not tolerate the procedure. This represents the main cause of patient refusal when they are invited to undergo desensitization. Berges-Gimeno et al.25 (2003) found that 67% of patients benefited from the therapy with aspirin during a one-year follow-up and 14% discontinued the use of ASA due to side effects, whereas 11% discontinued due to pregnancy or elective surgery. It is important to emphasize its correct continuous use. If patients discontinue treatment, they cannot re-start the medication on their own, because of the risk of severe reactions.
In the present study, in the group of patients who discontinued desensitization, three of eight patients were unable to finish it, due to adverse reactions and worsening of asthma. The other three had uncontrolled asthma after less than one month using aspirin 625mg twice daily, which required discontinuation. There are still factors that cannot be predicted or are independent from medical action, such as what occurred with a patient with suspected dengue fever who had to interrupt the medication due to the risk of Reye's syndrome.
Desensitization is capable of altering the natural course of AERD26 and should be included in the treatment of patients with the disease27; however, it should not be considered as an option to replace a new surgical intervention. The main goal is to postpone a new surgery or even make it less aggressive, thereby decreasing the risks for the patient. Another goal is to lessen morbidity, through symptom improvement and decrease in acute episodes. Although this was not a randomized, double-blind, placebo-controlled trial, this study showed that aspirin desensitization is an effective option for patients with AERD who meet the indications for this procedure. It depends on an excellent doctor–patient relationship, with full commitment by the patient to use aspirin uninterruptedly; the patient must be very well advised regarding all potential risks/side effects and the fact that treatment cessation may result in the loss of all that has been gained, with great possibility of disease recurrence.
ConclusionDespite the small sample size, aspirin desensitization has shown to be an effective alternative treatment for patients with poor clinical response. More studies, performed for a longer period of time, are necessary to better evaluate the effectiveness of aspirin desensitization in patients with AERD.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Spies JW, Valera FCP, Cordeiro DL, de Mendonça TN, Leite MGJ, Tamashiro E, et al. The role of aspirin desensitization in patients with aspirin-exacerbated respiratory disease (AERD). Braz J Otorhinolaryngol. 2016;82:263–8.