Adenoid cystic carcinoma is the most frequent malignant tumor of the submandibular gland and the minor salivary glands. It is a malignant neoplasm that, despite its slow growth, shows an unfavorable prognosis.
ObjectivesThe aim of this study was to perform a systematic review of the literature on Adenoid cystic carcinoma in the head and neck region and its clinicopathological characteristics, with emphasis on the perineural invasion capacity of the tumor.
MethodsA systematic search of articles published between January 2000 and January 2014 was performed in the PubMed/MEDLINE, SciELO, Science Direct, and Scopus databases.
ResultsNine articles were selected for this systematic review. These demonstrated that the female gender was more often affected and that malignant tumors showed a high rate of distant metastasis, recurrence, and a low survival rate. The presence of perineural invasion ranged from 29.4% to 62.5% and was associated with local tumor recurrence.
ConclusionAdenoid cystic carcinoma is commonly characterized by the presence of pain, high rate of recurrence, metastasis, and a low survival rate. Reporting studies with patient follow-up is of utmost importance for a better clinical-pathological understanding and to improve the prognosis of this pathology.
O carcinoma adenoide cístico (CAC) é o tumor maligno mais frequente da glândula Submandibular e das glândulas salivares menores. Sendo uma neoplasia maligna, apesar de ter crescimento lento, apresenta um prognóstico desfavorável.
ObjetivosO objetivo deste trabalho foi realizar uma revisão sistemática de literatura sobre o carcinoma adenóide cístico na região de cabeça e pescoço e suas características clínico-patológicas com ênfase na capacidade de infiltração perineural do tumor.
MétodoUma busca sistemática de artigos publicados entre janeiro de 2000 a janeiro de 2014 foi executada nas bases de dados PubMed/MEDLINE, SciELO, Science Direct e Scopus.
ResultadosNove artigos foram selecionados para realização da revisão sistemática. Nestes, o sexo feminino foi o mais afetado e o tumor maligno apresentou uma alta taxa de metástase a distância, recidiva e baixa taxa de sobrevida. A presença de invasão perineural variou entre 29,4% a 62,5% e foi relacionada à recidiva local do tumor.
ConclusãoO CAC é comumente caracterizado pela presença de dor, alta taxa de recidiva, metástase e baixa sobrevida. A realização de estudos com acompanhamento dos pacientes é de extrema importância para uma melhor avaliação clinico-patológica visando melhorar o prognóstico da doença.
Salivary gland neoplasms are unusual and account for only about 2–6.5% of tumors of the head and neck region.1 The frequency of different types of malignant tumors varies according to the site of origin. However, adenoid cystic carcinoma (ACC) appears to be the most common malignant tumor of the submandibular salivary and minor salivary glands.2
The ACC is a malignant neoplasm that, in spite of its slow growth, has a poor prognosis due to aggressive tumor invasion and a high rate of recurrence.3 This neoplasm was first described by Billroth in 1856.4
It can develop in several anatomical sites, such as the major and minor salivary glands, lacrimal glands, and upper aerodigestive tract glands.5 In the buccal-maxillofacial region, it accounts for approximately 22% of neoplasms of major and minor salivary glands, with the minor salivary glands of the hard palate representing the main affected site.2
ACC is more prevalent in middle-aged adults and according to many studies, it is more frequent in the female gender.5,6 Distant metastasis is common; the lung is the most commonly affected site.7
It may present clinically as a hardened lump, and pain is an important and common finding in the initial course of the disease. An ulcerated lesion can be seen on the palate, with bone destruction identified radiographically.8
Histopathologically, ACC can manifest as different types, with three main recognized patterns9: the cribriform and tubular patterns, which are less aggressive, and the solid pattern, which shows cell pleomorphism and mitotic activity, as well as necrotic foci in the central region of neoplastic cell islands.5,6
Perineural invasion is a common histological finding, and is considered a possible route for tumor cell dissemination.5 Perineural involvement occurs in approximately 22–46% of cases of ACC, whether at macro- or microscopic level.5
Surgery is the treatment of choice for ACC and may be followed by radiation therapy and, in rare cases, chemotherapy. The frequency of local recurrence for ACC is high, requiring additional surgical resections. Several studies have evaluated this development as a negative one, with the tumor causing the patient's death.9
Dental surgeons need to detect any changes in the oral mucosa of their patients. Early diagnosis of ACC results in better quality of life and higher survival rate. The objective of this study was to perform a systematic review of literature on ACC of the head and neck region and its clinical and pathological features, with emphasis on tumor perineural invasion capacity.
MethodsA systematic search for articles published between January 2000 and January 2014 was conducted in the PubMed/MEDLINE, SciELO, Science Direct, and Scopus databases. Studies that assessed ACC of the head and neck region and its perineural invasion capacity were evaluated.
The following terms were used in the search: adenoid cystic carcinoma; neoplasm; salivary gland, and perineural invasion, as well as their synonyms and corresponding terms in Portuguese and Spanish, in several combinations. Boolean operators AND, OR, NOT were used when possible. After obtaining the summaries, three independent evaluators selected the relevant studies according to the inclusion and exclusion criteria.
The inclusion criteria were: studies published in English, Portuguese, or Spanish; cross-sectional and longitudinal studies aimed at evaluating ACC of the head and neck region and its perineural invasion capacity; articles published from January of 2000 onward. The exclusion criteria were: review articles, population that did not match the research standards, clinical cases, and articles published before the year 2000 or in a different language from those selected for this systematic review.
The first step in study selection was analysis of titles and abstracts. Subsequently, all studies whose titles or abstracts were considered relevant were obtained and analyzed in full; finally, the articles analyzed and selected by the evaluators after a consensus meeting were included in the systematization of data.
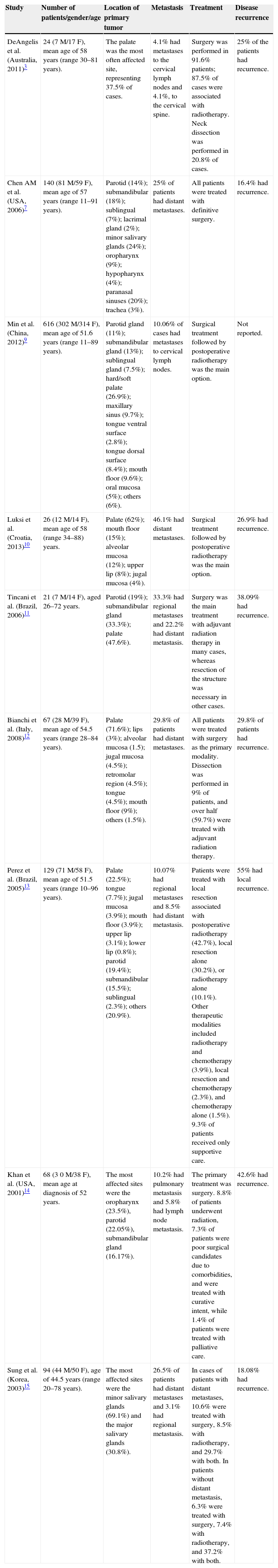
ResultsAmong the initially selected studies, 29 showed potential to be included in the systematic review; however, after full analysis of the studies and discussion of their contents by the evaluators, they agreed that only nine articles met all inclusion criteria.3,7,9–15 Among the selected studies, three were carried out in developing countries9,11,13 and six in developed ones.3,7,10,12,14,15 Article methods and results are shown in Tables 1 and 2.
Results obtained by the selected studies.
| Study | Number of patients/gender/age | Location of primary tumor | Metastasis | Treatment | Disease recurrence |
|---|---|---|---|---|---|
| DeAngelis et al. (Australia, 2011)3 | 24 (7 M/17 F), mean age of 58 years (range 30–81 years). | The palate was the most often affected site, representing 37.5% of cases. | 4.1% had metastases to the cervical lymph nodes and 4.1%, to the cervical spine. | Surgery was performed in 91.6% patients; 87.5% of cases were associated with radiotherapy. Neck dissection was performed in 20.8% of cases. | 25% of the patients had recurrence. |
| Chen AM et al. (USA, 2006)7 | 140 (81 M/59 F), mean age of 57 years (range 11–91 years). | Parotid (14%); submandibular (18%); sublingual (7%); lacrimal gland (2%); minor salivary glands (24%); oropharynx (9%); hypopharynx (4%); paranasal sinuses (20%); trachea (3%). | 25% of patients had distant metastases. | All patients were treated with definitive surgery. | 16.4% had recurrence. |
| Min et al. (China, 2012)9 | 616 (302 M/314 F), mean age of 51.6 years (range 11–89 years). | Parotid gland (11%); submandibular gland (13%); sublingual gland (7.5%); hard/soft palate (26.9%); maxillary sinus (9.7%); tongue ventral surface (2.8%); tongue dorsal surface (8.4%); mouth floor (9.6%); oral mucosa (5%); others (6%). | 10.06% of cases had metastases to cervical lymph nodes. | Surgical treatment followed by postoperative radiotherapy was the main option. | Not reported. |
| Luksi et al. (Croatia, 2013)10 | 26 (12 M/14 F), mean age of 58 (range 34–88) years. | Palate (62%); mouth floor (15%); alveolar mucosa (12%); upper lip (8%); jugal mucosa (4%). | 46.1% had distant metastases. | Surgical treatment followed by postoperative radiotherapy was the main option. | 26.9% had recurrence. |
| Tincani et al. (Brazil, 2006)11 | 21 (7 M/14 F), aged 26–72 years. | Parotid (19%); submandibular gland (33.3%); palate (47.6%). | 33.3% had regional metastases and 22.2% had distant metastasis. | Surgery was the main treatment with adjuvant radiation therapy in many cases, whereas resection of the structure was necessary in other cases. | 38.09% had recurrence. |
| Bianchi et al. (Italy, 2008)12 | 67 (28 M/39 F), mean age of 54.5 years (range 28–84 years). | Palate (71.6%); lips (3%); alveolar mucosa (1.5); jugal mucosa (4.5%); retromolar region (4.5%); tongue (4.5%); mouth floor (9%); others (1.5%). | 29.8% of patients had distant metastases. | All patients were treated with surgery as the primary modality. Dissection was performed in 9% of patients, and over half (59.7%) were treated with adjuvant radiation therapy. | 29.8% of patients had recurrence. |
| Perez et al. (Brazil, 2005)13 | 129 (71 M/58 F), mean age of 51.5 years (range 10–96 years). | Palate (22.5%); tongue (7.7%); jugal mucosa (3.9%); mouth floor (3.9%); upper lip (3.1%); lower lip (0.8%); parotid (19.4%); submandibular (15.5%); sublingual (2.3%); others (20.9%). | 10.07% had regional metastases and 8.5% had distant metastasis. | Patients were treated with local resection associated with postoperative radiotherapy (42.7%), local resection alone (30.2%), or radiotherapy alone (10.1%). Other therapeutic modalities included radiotherapy and chemotherapy (3.9%), local resection and chemotherapy (2.3%), and chemotherapy alone (1.5%). 9.3% of patients received only supportive care. | 55% had local recurrence. |
| Khan et al. (USA, 2001)14 | 68 (3 0 M/38 F), mean age at diagnosis of 52 years. | The most affected sites were the oropharynx (23.5%), parotid (22.05%), submandibular gland (16.17%). | 10.2% had pulmonary metastasis and 5.8% had lymph node metastasis. | The primary treatment was surgery. 8.8% of patients underwent radiation, 7.3% of patients were poor surgical candidates due to comorbidities, and were treated with curative intent, while 1.4% of patients were treated with palliative care. | 42.6% had recurrence. |
| Sung et al. (Korea, 2003)15 | 94 (44 M/50 F), age of 44.5 years (range 20–78 years). | The most affected sites were the minor salivary glands (69.1%) and the major salivary glands (30.8%). | 26.5% of patients had distant metastases and 3.1% had regional metastasis. | In cases of patients with distant metastases, 10.6% were treated with surgery, 8.5% with radiotherapy, and 29.7% with both. In patients without distant metastasis, 6.3% were treated with surgery, 7.4% with radiotherapy, and 37.2% with both. | 18.08% had recurrence. |
M, male; F, female.
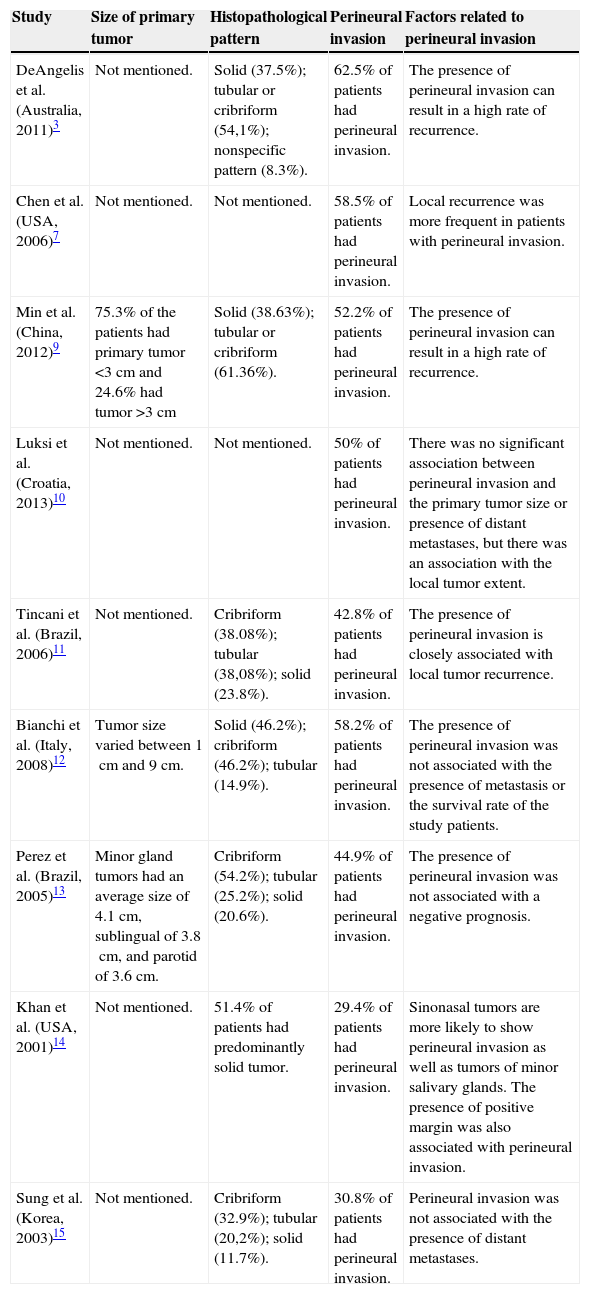
Tumor characteristics and perineural invasion.
| Study | Size of primary tumor | Histopathological pattern | Perineural invasion | Factors related to perineural invasion |
|---|---|---|---|---|
| DeAngelis et al. (Australia, 2011)3 | Not mentioned. | Solid (37.5%); tubular or cribriform (54,1%); nonspecific pattern (8.3%). | 62.5% of patients had perineural invasion. | The presence of perineural invasion can result in a high rate of recurrence. |
| Chen et al. (USA, 2006)7 | Not mentioned. | Not mentioned. | 58.5% of patients had perineural invasion. | Local recurrence was more frequent in patients with perineural invasion. |
| Min et al. (China, 2012)9 | 75.3% of the patients had primary tumor <3cm and 24.6% had tumor >3cm | Solid (38.63%); tubular or cribriform (61.36%). | 52.2% of patients had perineural invasion. | The presence of perineural invasion can result in a high rate of recurrence. |
| Luksi et al. (Croatia, 2013)10 | Not mentioned. | Not mentioned. | 50% of patients had perineural invasion. | There was no significant association between perineural invasion and the primary tumor size or presence of distant metastases, but there was an association with the local tumor extent. |
| Tincani et al. (Brazil, 2006)11 | Not mentioned. | Cribriform (38.08%); tubular (38,08%); solid (23.8%). | 42.8% of patients had perineural invasion. | The presence of perineural invasion is closely associated with local tumor recurrence. |
| Bianchi et al. (Italy, 2008)12 | Tumor size varied between 1cm and 9cm. | Solid (46.2%); cribriform (46.2%); tubular (14.9%). | 58.2% of patients had perineural invasion. | The presence of perineural invasion was not associated with the presence of metastasis or the survival rate of the study patients. |
| Perez et al. (Brazil, 2005)13 | Minor gland tumors had an average size of 4.1cm, sublingual of 3.8cm, and parotid of 3.6cm. | Cribriform (54.2%); tubular (25.2%); solid (20.6%). | 44.9% of patients had perineural invasion. | The presence of perineural invasion was not associated with a negative prognosis. |
| Khan et al. (USA, 2001)14 | Not mentioned. | 51.4% of patients had predominantly solid tumor. | 29.4% of patients had perineural invasion. | Sinonasal tumors are more likely to show perineural invasion as well as tumors of minor salivary glands. The presence of positive margin was also associated with perineural invasion. |
| Sung et al. (Korea, 2003)15 | Not mentioned. | Cribriform (32.9%); tubular (20,2%); solid (11.7%). | 30.8% of patients had perineural invasion. | Perineural invasion was not associated with the presence of distant metastases. |
Regarding the study profiles, sample size ranged from 2111 to 6169 patients, with the total sample size being 1185 patients with a mean age >50 years. The selected studies were published between the years 2001 and 2013. All of the selected studies were retrospective.
The objectives of the selected studies were: to perform the analysis of the clinical and pathological characteristics of ACC, analyzing variables such as gender,3,7,9–15 age,3,7,9–15 metastasis,3,7,9–15 recurrence,3,7,10–15 perineural invasion capacity,3,7,9–15 survival rates,3,7,9–15 and treatment.3,7,9–15
Among the studies selected for this systematic review, seven showed difference between genders, with the female gender more susceptible to ACC.3,9–12,14,15 Only two studies showed a higher number of cases in males.7,13
The palate was the most often affected site in the majority of the selected studies,3,9–13 reaching 71.6% of cases in the study by Bianchi et al.12 In the study by Khan et al.,14 the most often affected site was the oropharynx, followed by the parotid and submandibular glands. Among the selected studies, all assessed the presence of metastasis (Table 1).
The presence of perineural invasion was a constant in the selected studies. In the retrospective analysis by Min et al.,9 328 patients had perineural invasion, with 40 patients showing positive proximal metastasis and 288 negative. Fifteen cases were recorded in the study by DeAngelis et al.3 and 13 cases in the study by Luksié et al.10 Tincani et al.11 reported nine cases of perineural invasion, whereas Bianchi et al.12 reported 39 cases. The retrospective study by Perez et al.13 reported the presence of 58 cases, whereas Chen et al.7 reported 82 cases. Khan et al.14 reported the presence of 20 cases of perineural invasion, while Sung et al.15 reported 29 patients, 12 with positive distant metastases and 17 negative. In the study performed by Lukisi et al.,10 there was no significant association between the presence of perineural invasion and primary tumor size, presence of proximal or distant metastasis, or invasion of margins; however, it was associated with local tumor extent.
Among the mentioned treatments, the most frequently performed were surgical excision1–23 and radiotherapy,3,9–15 with treatment by chemotherapy also reported.13 Among the studies that mentioned the presence of recurrence3,7,10–15 until the time of publication of these articles, the study by Perez et al.13 showed the highest number of cases, with a total of 71 patients (55%) with recurrence. Min et al.9 does not mention the presence of recurrence. Overall survival rates were variable between studies. In the study by DeAngelis et al.,3 overall survival rates at 5, 10, and 20 years were, respectively, 92%, 72%, and 54%. In the study by Min et al.,9 the survival rate was correlated with the occurrence of metastases, with an overall five-year survival in patients with lymph node metastasis of 48%, and in those without lymph node metastasis, 77%. In the study by Luksi et al.,10 survival rates were 62% at five years, 53% at 10 years, and 27% at 15 years.
DiscussionThe studies selected in this systematic review evaluated several aspects of ACC in the head and neck region. Among the selected studies, seven showed that the malignant tumors were more frequently found among the female population,3,10–15 whereas two studies showed the tumors were more frequent in the male gender.7,9 In spite of the divergence, the scientific literature demonstrates a greater susceptibility in women.16
In the study by Perez et al.,13 the most common clinical signs and symptoms were the presence of nodular enlargement (92.1%), pain (59.8%), paresthesia (12.6%) and nasal congestion (11.8%). In the analysis by Bianchi et al.,12 primary tumor size varied between 1cm and 9cm. Studies have shown that tumors >3cm may have a high metastatic rate, apart from other factors such as gender, age, and perineural invasion.17,18
Among the studies that evaluated histopathological types, the most frequent was the cribriform pattern, followed by tubular and solid patterns.3,9,11,13,15 In the study by Khan et al.,14 there was a predominance of the solid pattern, whereas in the study by Bianchi et al.,12 the number of affected cases showing the cribriform and solid patterns were similar.
The palate was the most often affected site in the majority of the selected studies, which is consistent with other studies.1,2,4 Only the study by Khan et al.14 mentions the oropharynx as the most often affected site. A study performed in 2007 shows that tumor location in the minor salivary glands may favor tumor recurrence and a worse prognosis.18
All selected studies analyzed the presence of metastases in patients with ACC. In the study by Sung et al.,15 which assessed the predictive factors and the impact of distant metastases in ACC in a total of 94 patients, 25 had distant metastases, while three had regional metastasis. Other studies have shown similar results, where the most common sites of metastasis were the lungs (80%), bone (15%), and liver and other sites (5%); these metastases may appear even 20 years after primary tumor resection, representing the major cause of treatment failure.19,20 However, among the selected studies, there were similarities between cases of distant and regional metastasis.11,13
Recurrence is frequent among patients with ACC.3,7,10–15 Tumor location in the minor salivary glands favors recurrence and appears to be associated with the more advanced cases and worse prognosis.18 In the study by Khan et al.,14 29 patients had local recurrence and the author associated the presence of perineural invasion with increased recurrence rates, as well as a higher incidence of positive margins, which is consistent with the other selected studies.3,7,9,11 A study conducted in 1997 found a decrease in recurrence rates over the years due to the effects of radiotherapy use, but with no significant impact on distant metastasis rates.17
Pain and discomfort complaints are not unusual and are often attributed to tumor invasive behavior. Tumor cells tend to invade and disseminate peripherally to the nerve fascicles. Perineural invasion is a common histological finding, and is considered a possible route for tumor cell dissemination.5,18 In the study by Khan et al.,14 perineural invasion was associated with higher recurrence rate and positive surgical margins; however, the study by Perez et al.13 did not show similar results. None of the studies associated the presence of perineural invasion to metastasis or survival rate; however, studies have shown that patients with perineural invasion require radiotherapy.21,22
The most commonly used treatment modality was surgical, whether or not followed by radiotherapy. In the study by de DeAngelis et al.,3 neck dissection was performed in five cases, whereas for Khan et al.,14 this treatment was used only in cases that showed some clinical or imaging sign that justified its use, considering the low rate of nodal metastatic involvement. Perez et al.13 also reported cases treated with associated chemotherapy; however, the importance of adjuvant chemotherapy after surgery has not been systematically explored.23
The survival rate varied among the selected studies. In the study by DeAngelis et al.,3 overall survival rates were 92% at five years, 72% at 10 years, and 54% in 20 years. In the study by Luksic et al.,14 survival rates were 62% at five years, 53% at 10 years, and 27% at 15 years. For Min et al.,9 life expectancy was directly associated with the occurrence of metastases and the overall five-year survival of patients with lymph node metastasis was 48%, whereas it was 77% in patients without metastasis. Perez et al.13 mentioned that the solid pattern has a lower survival rate; the impact of the solid tumors on survival can be explained, in part, by their greater metastatic potential. DeAngelis et al.3 demonstrated that patient survival rate decreases considerably in series with a follow-up lasting more than 15 years.
ConclusionACC is a malignant neoplasm of glandular tissue that has a higher incidence in females and is commonly characterized by the presence of pain, high rate of recurrence, metastasis, and poor survival. The presence of perineural invasion was not associated with metastasis or survival rate, but was associated with the presence of local recurrence and margin involvement, in addition to influencing appropriate patient treatment. Studies with long-term follow-up of patients to evaluate the clinical aspects of the tumor are extremely important for better understanding of ACC, as well as better clinical-pathological evaluation in order to improve its prognosis.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Dantas AN, de Morais EF, Macedo RA, Tinôco JM, Morais ML. Clinicopathological characteristics and perineural invasion in adenoid cystic carcinoma: a systematic review. Braz J Otorhinolaryngol. 2015;81:329–35.
Institution: Universidade Potiguar (UnP), Natal, RN, Brazil.