Upper airway nerve and muscle damage associated with obstructive sleep apnea may impair the strength and dynamics of pharyngeal and esophageal contractions during swallowing.
ObjectiveTo evaluate the presence of alterations in pharyngoesophageal manometry in patients with obstructive sleep apnea with and without oropharyngeal dysphagia.
MethodsThis study prospectively evaluated 22 patients with obstructive sleep apnea without spontaneous complaints of dysphagia, using a questionnaire, fiberoptic endoscopic evaluation of swallowing, and pharyngoesophageal manometry, including measurement of the upper and lower esophageal sphincter pressures and mean pharyngeal pressures at three levels during swallowing.
ResultsThe dysphagia group consisted of 17 patients (77.3%) in whom swallowing abnormalities were detected on fiberoptic endoscopic evaluation of swallowing (n=15; 68.2%) and/or in the questionnaire (n=7; 31.8%). The five remaining cases comprised a control group without oropharyngeal dysphagia. In all cases of abnormalities on fiberoptic endoscopic evaluation of swallowing, there was premature bolus leakage into the pharynx. There was no statistically significant difference between the groups regarding any of the pharyngoesophageal manometry measurements, age, or severity of obstructive sleep apnea.
ConclusionPharyngoesophageal manometry detected no statistically significant difference between the groups with and without oropharyngeal dysphagia.
Lesões neurogênicas e musculares associadas à apneia obstrutiva do sono podem comprometer a força e a dinâmica das contrações faríngeas e esofágicas durante a deglutição.
ObjetivoVerificar se há alterações na manometria faringoesofágica de pacientes com apneia obstrutiva do sono com e sem disfagia orofaríngea.
MétodoForam avaliados, prospectivamente, 22 pacientes com apneia obstrutiva do sono sem queixa espontânea de disfagia, utilizando questionário, videoendoscopia da deglutição e manometria faringoesofágica, com medidas das pressões do esfíncter esofagiano inferior e superior e pressão média da faringe em três níveis durante a deglutição.
Resultados17 pacientes (77,3%) formaram o grupo com disfagia, por apresentarem alterações de deglutição na videoendoscopia da deglutição (n=15; 68,2%) e/ou no questionário (n=7; 31,8%). Os cinco restantes compuseram o grupo sem disfagia orofaríngea. Em todos os casos com alterações na videoendoscopia da deglutição houve escape precoce do bolo alimentar para a faringe. Não houve diferença significante entre os grupos com e sem disfagia em relação a todas as medidas de manometria, idade e gravidade da apneia obstrutiva do sono.
ConclusõesA manometria faringoesofágica não demonstrou diferença significante entre os grupos com e sem disfagia orofaríngea.
Neural lesions in the soft palate and oropharynx are some of the alterations found in patients with obstructive sleep apnea (OSA) and primary snorers.1–3 Some authors believe that these lesions are triggered by low frequency vibrations produced by snoring or intermittent hypoxia related to OSA.4–6 The soft palate mucosa in primary snorers with OSA shows an increased number of abnormal nerve endings.1 The palatopharyngeal muscle, both in primary snorers and in patients with OSA, shows morphological alterations that are typical of peripheral nerve injury, such as grouping of tissues by fiber type, clusters of atrophied areas, and fascicular atrophy.2
The presence of neurological disorders in the pharynx of patients with OSA can cause swallowing process dysfunction, as the initiation of the swallowing reflex and propagation of the food bolus depends on adequate sensitivity (afferent) and pharyngeal function. Additionally, it is believed that the perpetuation of OSA impairs neuromuscular afferent stimulation of the upper airways and the central integration between swallowing and breathing functions.7–12
The evaluation of swallowing using videofluoroscopy or fiberoptic nasal endoscopy shows a high prevalence of alterations in patients with primary snoring or OSA. These alterations can be symptomatic or asymptomatic and consist mostly of premature bolus leakage (from the oral cavity into the pharynx) and food residue in the pharynx after swallowing.9,10,13,14
Pharyngoesophageal manometry assesses the compressive muscle force of the pharyngeal and esophageal muscles during swallowing, aiding in the understanding of the physiopathology of oropharyngeal dysphagia.15–17 Hypothetically, neurological and muscular disorders of the upper airways associated with OSA18 can impair the force and dynamics of pharyngoesophageal contractions during swallowing, contributing to the dysphagia observed in many cases of OSA. To the best of the authors’ knowledge, no studies have performed manometric evaluations of the pharyngeal phase of swallowing in patients with OSA.
The objective of this study of patients with OSA, was to evaluate whether swallowing pressures in the pharynx and esophagus are lower in patients with oropharyngeal dysphagia compared to those without oropharyngeal dysphagia.
MethodsMethods SubjectsWe evaluated twenty-two consecutive adult snorers with OSA (aged >18 years) who had been selected for pharyngeal surgical treatment for OSA in this institution. There was no patient loss. All patients had refused non-surgical treatment for OSA and were eligible for surgical pharynx expansion through repositioning of the muscle flaps on the pharyngeal lateral wall.19 Patients who had undergone any previous pharyngeal surgery, previous treatment for OSA, or those with clinically known dysphagia were excluded. This study also excluded patients with neuromuscular or rheumatological disease, Down syndrome, acquired or syndromic facial deformities, use of drugs that interfered with muscle tone, previous esophageal surgery, and those with symptoms of gastroesophageal reflux disease. This study is part of a research protocol to evaluate the effect of pharyngeal surgery on swallowing. It was approved by the Ethics Committee of this institution (protocol 003.0.388.000-10), registered in Clinical Trials (NCT01335594), and all patients signed an informed consent.
The diagnosis of OSA was based on the presence of characteristic symptoms and findings from a supervised overnight polysomnography evaluation carried out in the sleep laboratory. The patient group consisted of 17 men and five women, with a mean age of 48.4 years (range 27–62 years), body mass index (BMI) of 29.0kg/m2 (ranging from 25 to 35.1kg/m2), and mean neck circumference of 41.5cm (range 36–48cm). Six patients (27.3%) had systemic arterial hypertension and three were smokers (13.6%). Regarding the pharyngeal anatomy, 19 had grade I or II palatine tonsils (8.4%) and three had grade III or IV tonsils (13.6%).20
The mean apnea–hypopnea index (AHI) was 40.7 (range 7.2–89.4), with 16 patients (72.7%) with severe AHI (AHI >30), three (13.6%) with moderate AHI (AHI≤15≤30), and three (13.6%) with mild AHI (5≤AHI<15). The mean minimum oxyhemoglobin saturation was 77.6% (range 51–88%). The Epworth sleepiness scale (ESS) showed a mean score of 15 in this series, ranging from 4 to 19, with nine cases (40.9%) showing excessive sleepiness (Epworth >10).
The patients were divided into two groups, those with dysphagia and those without dysphagia, based on the assessment of the swallowing questionnaire and videoendoscopy of swallowing (VES). Patients were considered as dysphagic when they showed alterations in the questionnaire and/or VES. In contrast, patients with normal VES and questionnaire comprised the group without dysphagia.
QuestionnaireAll patients answered a questionnaire13 (Table 1) that included six questions about symptoms of dysphagia perceived by the patient in the previous month. The responses were scored using a scale of 0–3, where 0 meant “never”, 1 “rarely”, 2 “often”, and 3 “always”. A score of 2 or 3 in at least one of the questions was considered as indicative of the presence of dysphagia.
Swallowing assessment questionnaire. Score: 0=never, 1=rarely, 2=often, 3=always. The presence of scores 2 or 3 at any question signifies clinically present dysphagia.
| Questions | Score | |||
|---|---|---|---|---|
| 1. Do you choke on liquids during meals? | 0 | 1 | 2 | 3 |
| 2. Do you choke on semi-solid food during meals? | 0 | 1 | 2 | 3 |
| 3. Do you choke on solid food during meals? | 0 | 1 | 2 | 3 |
| 4. Do you feel the food went down the wrong place? | 0 | 1 | 2 | 3 |
| 5. Do you feel the food stuck in your throat? | 0 | 1 | 2 | 3 |
| 6. Do you feel the food is coming back through your nose? | 0 | 1 | 2 | 3 |
VES was performed21 in all patients, using a 3.2-mm flexible endoscope (Pentax – Japan), introduced through the wider nasal cavity without the use of topical anesthetic in order not to alter the upper airway mucosa sensitivity. The patient remained in a comfortable sitting position, with mild ventroflexion simulating a meal position, while the pharynx and larynx were assessed.
At the basal evaluation, the upper aerodigestive tract anatomy was assessed, as well as the presence of salivary stasis, laryngeal sensitivity (test of glottal adduction to endoscope touch), mobility, and appearance of the vocal folds and velopharyngeal closure during phonation and swallowing of saliva.
Under direct visualization through the endoscope, the dynamic assessment was performed for each patient during the swallowing of foods colored with blue food coloring, at room temperature, administered separately at three different consistencies (liquid, semi-solid, and solid). The complete swallowing of each consistency was assessed three times, totaling nine swallowing analyses. The liquid consistency comprised 5mL, 10mL, and 15mL of artificially flavored strawberry drink (Clight brand powdered soft drink, strawberry flavor; Kraft Foods Brazil SA – Curitiba, PR, Brazil) at each time.
The semi-solid food consisted of artificially flavored strawberry mixed with a thickening agent (BioSen NutriSenior®, Taboão da Serra, SP, Brazil), administered three times using a tablespoon. For solid food, three 2.5cm×2.5cm crackers were given to patients.
The examinations were recorded on DVD for reanalysis. The authors classified the following parameters as present or absent: (a) premature leakage: the food bolus leaves the oral cavity and reaches the pharynx before the swallowing reflex is triggered; (b) velopharyngeal dysfunction: the soft palate does not fully occlude the nasopharynx during swallowing, allowing food leakage; (c) laryngeal penetration: the food enters the larynx, but does not cross the glottis; (d) tracheal aspiration: the food enters the larynx and goes through the glottis; (e) food residue after swallowing: presence of some food in the pharynx after three complete swallowing movements. The presence of premature leakage was considered when it occurred in at least two of the nine swallowing analyses; as for to the other alterations, their occurrence in one series was sufficient to be classified as present.
Additionally, we evaluated whether the cough reflex was associated with the penetration and/or aspiration events and whether it was effective in eliminating the penetrated and/or aspirated content, as well as the number of spontaneous or requested swallowing movements necessary for complete clearing of the food bolus (which was considered abnormal when there were more than three).
The presence of any alteration in the dynamic assessment placed the patient in the dysphagia group. Dysphagia severity was not assessed.
Pharyngoesophageal manometryPharyngoesophageal manometry was performed in all patients by the same examiner, who was blinded to patients’ group assignment. An eight-channel computer polygraph (Alacer®, Brazil) was used, connected to a catheter (4mm diameter) with four longitudinal and four radial channels under a pneumohydraulic capillary infusion through the perfusion method in a low-compliance system with a continuous flow of 0.6mL/min/channel. All tests were performed with patients in the supine position. Before examinations, patients were instructed to fast for 6h prior to the test. The basal pressures (without swallowing) of the lower (LES) and upper (UES) esophageal sphincter were evaluated, as well as intrapharyngeal pressures during swallowing.
The catheter was introduced through the nose into the stomach, which was located by assessing the pressure measured by the device. Then, the LES was assessed using the slow withdrawal technique, i.e., pulling out the catheter 1cm at a time with the nostril as the point of reference. The zero reference of the examination is the intragastric pressure, which is the gastric baseline. The pressures increase in the LES and decrease again in the esophageal body (esophageal baseline), increasing again in the UES. The pressures in the LES are higher during inspiration and lower during expiration.
The LES pressure, obtained in the region with higher pressures over three to five stable respiratory cycles, was calculated as the difference between the intragastric pressure and the maximum LES pressure during expiration, and considered by the simple arithmetic mean of pressures in each of the four channels on the catheter tip, yielding the maximal expiratory pressure (MEP). The mean LES pressure between expiration and inspiration corresponds to the mean respiratory pressure (MRP). Values below the normal MEP and/or MRP values indicate LES hypotonia. The relaxation of the LES was assessed during wet swallowing.22,23
For the study of UES, the authors also used the four more distal catheter radial openings and the same slow withdrawal technique. The resting pressure of the UES was analyzed, and represented the difference between the esophageal baseline and the manometric tracing. This was measured at the point of the highest and most stable pressure. The relaxation of the UES was evaluated at the point of highest pressure, with three swallowing movements of 5mL of water.24
The pharynx was evaluated with the radial channels in three different points, located 2cm, 4cm, and 6cm above the upper border of the UES. The amplitude of pharyngeal contraction was determined, which represented to the difference between the pharyngeal baseline and the maximum wave peak studied. For that purpose, swallowing pressure measurements were conducted three times at each point, with the intake of 5mL of water, and considering the mean value of the three swallowing movements at each point (mean pharyngeal pressure – MPP).
Statistical analysisSpearman's correlation was used to study the association between quantitative variables. Regarding the qualitative variables, Fisher's exact test was used to verify associations. Wilcoxon's rank-sum test was used to study the association between qualitative and quantitative variables. The 95% confidence interval for proportions was calculated for qualitative variables. The significance level used in the tests was 5%, always considering a two-tailed alternative hypothesis.
ResultsOf the 22 patients assessed, 17 (77.3%) had dysphagia; two (9.1%) due to alterations detected only in the swallowing questionnaire, 10 (45.5%) due to alterations in the VES, and five (22.7%) with alterations in both the VES and the questionnaire. The dysphagia group had a mean age of 48.6 years, with 12 men (70.6%), a mean AHI of 41.7/h, a mean BMI of 28.6kg/m2, and a mean neck circumference of 40.9cm. The non-dysphagia group (five cases; 22.7%) had a mean age of 47.6 years (p=0.70), with five men (100%) (p=0.29), a mean AHI of 37/h (p=1.00), a mean BMI=30.4kg/m2 (p=0.32), and a mean neck circumference of 43.5cm (p=0.10).
Among the seven cases with dysphagia complaints (31.8%), the following were observed in the swallowing questionnaire: two mentioned choking with liquids, one with semi-solid, and three with solid food; three reported the feeling that the food “had gone down the wrong place”, and six reported the feeling of “food stuck in the throat”. There were no complaints of nasal reflux of food or liquids.
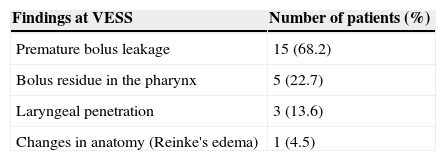
The VES (Table 2) showed alterations in 15 of 22 patients (68.2%). When analyzing the anatomy of the upper aerodigestive tract, a mild case of Reinke's edema was detected. The most common finding was the premature bolus leakage into the pharynx before the swallowing reflex was triggered, which occurred in all cases with altered VES. There were no cases of tracheal aspiration or velopharyngeal insufficiency.
Alterations found in the Fiberoptic endoscopic evaluation of swallowing (FEES) in the 22 patients studied (total of 15 cases with alterations).
| Findings at VESS | Number of patients (%) |
|---|---|
| Premature bolus leakage | 15 (68.2) |
| Bolus residue in the pharynx | 5 (22.7) |
| Laryngeal penetration | 3 (13.6) |
| Changes in anatomy (Reinke's edema) | 1 (4.5) |
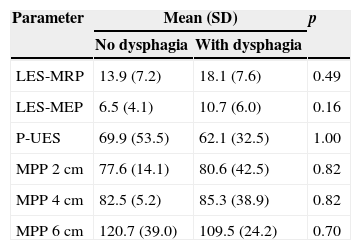
There was no difference between the groups with and without oropharyngeal dysphagia in relation to all pharyngoesophageal manometry measurements (Table 3). The dysphagia group had eight cases (47.1%) of LES hypotonia, two cases (11.8%) of UES hypertonia, and one case (5.9%) of UES hypotonia, but with normal UES relaxation and coordinated with the pharynx in all cases. In the non-dysphagia group, there were four cases (80%) of LES hypotonia (p=0.32) and one case (20%) of UES hypertonia (p=1.00), also with normal UES relaxation and coordinated with the pharynx in all cases.
Comparison between groups with and without dysphagia regarding manometric measurements in the lower esophageal sphincter (LES), upper esophageal sphincter (UES), and the pharynx at the levels of 2cm; 4cm, and 6cm above the UES.
| Parameter | Mean (SD) | p | |
|---|---|---|---|
| No dysphagia | With dysphagia | ||
| LES-MRP | 13.9 (7.2) | 18.1 (7.6) | 0.49 |
| LES-MEP | 6.5 (4.1) | 10.7 (6.0) | 0.16 |
| P-UES | 69.9 (53.5) | 62.1 (32.5) | 1.00 |
| MPP 2 cm | 77.6 (14.1) | 80.6 (42.5) | 0.82 |
| MPP 4cm | 82.5 (5.2) | 85.3 (38.9) | 0.82 |
| MPP 6cm | 120.7 (39.0) | 109.5 (24.2) | 0.70 |
LES, lower esophageal sphincter; MRP, mean respiratory pressure; MEP, maximal expiratory pressure; P, pressure; UES, upper esophageal sphincter; MPP, mean pharyngeal pressure; SD, standard deviation.
The correlation between the variables UES, LES, mean pharyngeal pressure, questionnaire results, and VES findings, as well as between them and the variables age and AHI were assessed. The only statistically significant finding was the correlation between laryngeal penetration and age. Patients with laryngeal penetration were older (mean age 60 years, SD=0.00) compared to those without laryngeal penetration (mean age 46.5 years, SD=9.7; p=0.01).
DiscussionIn this series of consecutive cases, a high frequency of dysphagia in the group of adult patients with OSA that were snorers was observed, with 31.8% of patients reporting symptoms of dysphagia and 45.5% showing altered VES without clinical symptoms. All 68.2% of cases with abnormal VES had premature leakage of the food bolus between the oral cavity and the pharynx. This may suggest an impairment in the afferent sensory function of the oropharyngeal mucosa or oral phase dysfunction, at the approximation of the posterior part of the tongue with the soft palate.
When premature leakage occurs, mastication and breathing are not inhibited, and thus, there is a risk of tracheal penetration or aspiration.25 The pharyngeal and esophageal manometry showed no differences between the groups with and without dysphagia, as well as the demographic, anthropometric, and polysomnographic data. These findings seem to favor the role of neurogenic alterations as the cause of oropharyngeal dysphagia in OSA, to the detriment of the role of pharyngeal muscle alterations.1,2 To the authors’ knowledge, this is the first study that measured the pharyngeal pressure of swallowing in patients with OSA.
Although no differences were found regarding the age of patients with and without alterations in VES, those with laryngeal penetration were older than those without it. Swallowing assessment through videofluoroscopy with barium showed that among snoring patients with OSA, dysphagia appeared in older patients, regardless of OSA severity.10 Studies in healthy individuals have shown that aging is associated with a higher frequency of penetration and aspiration compared to young adults, but without excluding patients with snoring and apnea.26,27 One hypothesis that could be raised is that older patients have had a longer exposure to vibration trauma caused by snoring, resulting in more severe swallowing alterations.
Although the present study favors the hypothesis of a peripheral cause for dysphagia in OSA, this subject is controversial in the literature. Teramoto et al. showed that the latency of the pharyngeal swallowing reflex was increased, which required a larger volume of food in the pharynx to trigger the reflex in patients with OSA.7 Jobin et al. found a significant reduction in the swallowing reflex latency in OSA, suggesting the impairment of the inhibitory modulation of the reflex and central control of swallowing.8
Snorers without9,10 and with OSA9,10,14 show subclinical swallowing alterations between 52% and 64% of cases, comparable to the 45.5% found in the present study. Apparently, the risk of dysphagia in snorers does not correlate with the presence or severity of OSA,10 indicating the harmful role of tissue vibration caused by snoring, leading to nerve damage in the upper airways, which would contribute to dysphagia.28 There was no correlation between the severity of OSA and the presence of dysphagia in the present study.
It was observed that, in general, the complaint of dysphagia was not spontaneously mentioned by patients with OSA, but when perceived, it alerted patients to other potential impacts of OSA on their quality of life, acting as an additional motivator for seeking and adhering to treatment. This aspect is often ignored in the management of OSA; specific treatments for swallowing, such as the teaching of maneuvers, postural adjustments, facilitation therapies, and changes in diet consistency can also have a positive impact on quality of life of these patients, similar to what occurs in patients with dysphagia secondary to Parkinson's disease.29,30
Among the 31.8% cases with dysphagia complaints, the majority (27.3%) reported having the sensation of food stuck in the throat. In clinical practice, this symptom is usually attributed to pharyngolaryngeal reflux. However, pharyngeal dysphagia is often a slowly progressive disorder, in which the individual develops compensatory mechanisms such as diet changes or mastication velocity. Thus, the symptoms may appear only when the compensatory strategies no longer overcome the intensity of the disorder. Prior to that, an active medical intervention can already detect impaired swallowing.31 Thus, the early and adequate treatment of snoring and OSA can prevent the development of pharyngeal dysphagia. The literature has a report of two cases of severe OSA that showed improvement of dysphagia after one year of treatment with continuous nasal positive airway pressure (CPAP) and weight loss.32
We found no differences in pharyngeal swallowing pressures between the assessed groups. Nonetheless, 22.7% of patients had food residue in the pharynx after completion of the swallowing movement and resumption of breathing, possibly indicating alterations in peristalsis or pharynx elevation, as the UES relaxation was not different between the groups. To the best of the authors’ knowledge, the normal values for pharyngeal pressure in this population are unknown, although there are initial studies with the Japanese population.33 As much of the pharyngeal swallowing pressure is exerted by the tongue, whose electromyographic activity is increased during wakefulness in patients with OSA,34 it is considered unlikely that pharyngeal manometry will show alterations in future studies of OSA cases, unlike previous studies with myasthenia gravis and Huntington's disease, which are characterized by major alterations in muscle strength.35,36
Esophageal pressure measures did not differ between the groups with and without dysphagia. The finding of LES hypotonia, which can be associated with gastroesophageal reflux disease (GERD),37 was present in both groups (p=0.32), but since it was an exclusion criteria, our patients had no symptoms suggestive of the disease. The association between GERD and OSA has been described in the literature,38,39 but was not evaluated in this study.
We recognize that this study has some limitations. A larger patient group without dysphagia perhaps could have revealed some statistical differences that were not demonstrated in this analysis. Additionally, the inclusion of a group of primary snorers and a control group neither snored snorers nor had OSA patients would help to clarify the roles of OSA and snoring in the dysphagia these patients exhibited, perhaps demonstrating differences in pharyngeal swallowing pressures between the groups. The use of a validated questionnaire would be more appropriate, but the study was based on a simpler questionnaire used in a similar study since these patients did not have many complaints of dysphagia. Another limitation was the fact that solid-state esophageal manometry which is superior when assessing rapid high-pressure events that occur in the pharynx was not available.15,33 However, the perfusion method was performed by an experienced gastroenterologist who personally conducted the examination in all patients.
ConclusionThis study found that among patients with OSA, there was no significant difference in swallowing pressures of the pharynx and esophagus between subjects with and without oropharyngeal dysphagia.
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Oliveira LA, Fontes LH, Cahali MB. Swallowing and pharyngo-esophageal manometry in obstructive sleep apnea. Braz J Otorhinolaryngol. 2015;81:294–300.
Institution: Hospital do Servidor Público Estadual de São Paulo, São Paulo, SP, Brazil.