To setup an experimental model is the first step to study neural regeneration. Aim: Setting up an experimental model on facial nerve regeneration. Material and Methods: Wistar rats with complete sectioning and suturing of the extratemporal facial nerve trunk; with a behavioral and histological analysis for 9 weeks. Study Design: Experimental prospective study. Results: Progressive clinical and histological recovery of the animals. Conclusion: Our method is acceptable to study facial nerve regeneration in rats.
The facial nerve -either of the seventh pair of cranial nerves- plays an important role in various bodily functions. It is responsible for homolateral hemifacial motion and assists in the motion of neck muscles, in the efferent stapedius reflex, in outer ear sensitivity, in gustatory functions and in the autonomic control of exocrine glands. Injuries to the facial nerve pose marked impact on anyone’s well-being, as cosmetic, functional, and psychological impairments ensue1–3.
Publications in the medical literature on attempts to repair injured facial nerves date back to the 17th century4, but the first successful account happened only in 1927 by Bunne15. During World War II, as patients poured in from the battle fields, efforts were initiated to treat neural lesions. Published in 1942, ’Senior Consultant in Neurologic Surgery to the European Theater of Operations’6 argued in favor of early tensionless sutures to improve functional outcomes. However, recovery results for injured facial nerves remain controversial1,2,7,8. Several papers have been published on the weight various factors bear on the recovery of lesioned facial nerves, encompassing surgical techniques2, delivery of neurotrophic agents1,7,9 and even exposure to electromagnetic pulses10. All such studies have led to the development of experimental methods to analyze injured facial nerves.
This paper aims to set a reproducible experimental paradigm to analyze total facial nerve lesions that allows for the analysis of nerve regeneration along time and facilitates the implementation of future studies on the use of neurotrophic factors.
MATERIALS AND METHODSThe research project described in this paper was reviewed and approved by the Research Ethics Committee at UNIFESP/EPM under protocol # 0418/05.
Adult male Wistar rats with weight ranging between 200 and 300 grams were used in the study. They were kept in proper cages, under controlled environmental conditions and 12-hour darkness/light cycles, with free access to water and feed. The rats were divided into six groups and underwent facial nerve sectioning followed by suturing, slaughtering and histology exams at 1, 3, 5, 7 and 9 weeks after surgery. The 9-week group (9 SEM) followed the algorithm described below:
D0 D01 D07 D14 D21 D28 D35 D42 D49 D56 D63
|____________|____|_____|____|____|____|____|____|____|______|
Initial surgery behavior observation slaughter
* Sectioning of nerve and suturing * nerve histology exams
Note: D01 (first day post-op), D07 (seventh day post-op), D14 (fourteenth day post-op)...
None of the other groups (0 SEM, 1 SEM, 3 SEM, 5 SEM and 7 SEM) were observed for behavior and were slaughtered as scheduled on D0, D7, D21, D35 and D49 respectively. On D0 all rats underwent surgery using a DF-Vasconcelos® M90 surgical microscope, as described below:
- 1)
Intraperitoneal anesthesia with 2% xylazine chloridrate (0.5 ml/Kg of weight) and 10% ketamine chloridrate (0.9 ml/Kg of weight);
- 2)
right-side retroauricular hair removal;
- 3)
Vertical retroauricular incision encompassing skin, subcutaneous tissue, and the platysma;
- 4)
blunt dissection and identification of the tendinous margin of the clavicle-trapezius muscle, the trunk and furcation of the right-side facial nerve and external jugular vein;
- 5)
Total sectioning of the facial nerve with micro scissors approximately 3mm above its point of emergence (between the tendinous margin of the clavicle-trapezius muscle and the external jugular vein) and followed by immediate suture with 9-0 nylon wire;
- 6)
Closure of skin and subcutaneous tissues by separate stitches using 4-0 nylon wire.
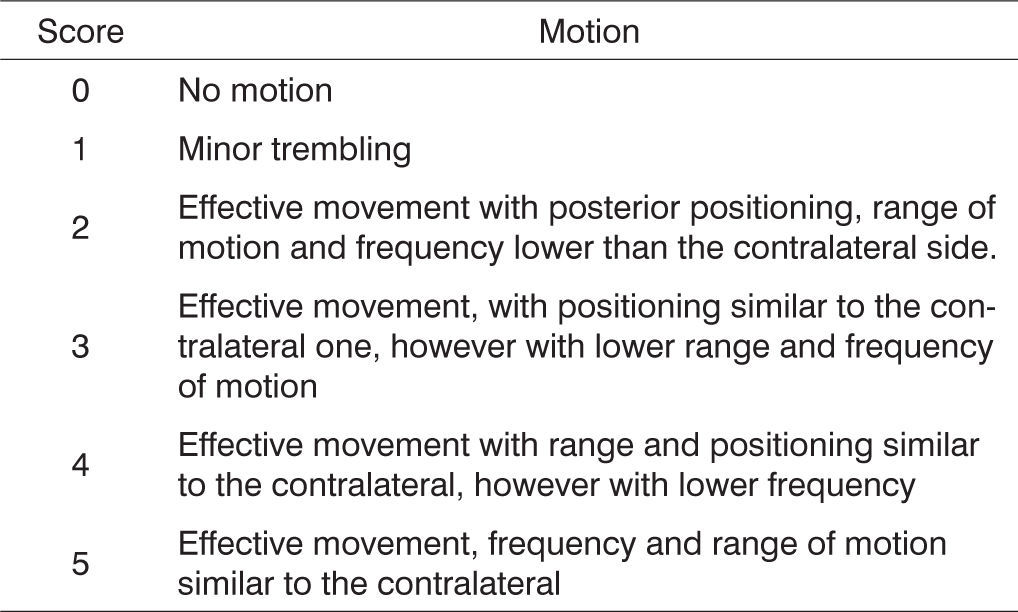
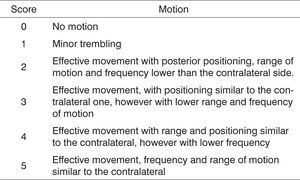
On the days set aside for clinical observation the rats were observed for spontaneous facial movement and motion of vibrissae, comparing the injured right side to the normal left side as rats went by exploring the grounds. Scores were assigned based on vibrissae position, frequency and range of motion as described on Figure 1.
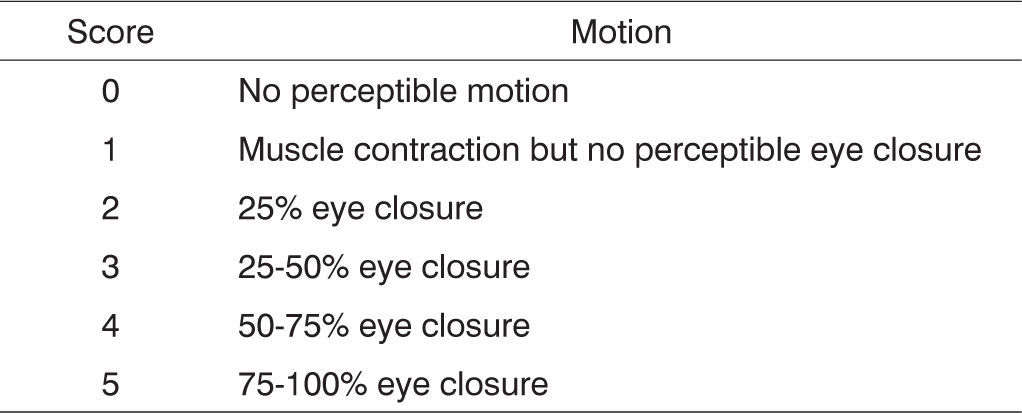
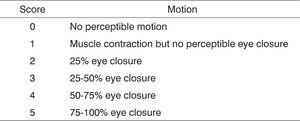
The rats’ eye-closure capability was also assessed during clinical observation through stimulation by a burst of air produced by quickly pushing on the plunger of a 20-ml syringe pointing to each of the animals’ eyeballs. No comparisons were made between injured (right) and normal (left) sides as the lateralized position of the rats’ eyeballs does not allow for simultaneous bilateral stimulation. Scores were assigned based on observed eyelid closure as described in Figure 2.
All rats were slaughtered on the days scheduled for their specific groups by the administration of a high dose of the same anesthetics used during initial surgery. The right-side facial nerves were once again dissected and the previous neural sutures located. The fragments immediately distal to the point of suture were removed for histologic examination. Nerve fragments were placed in individual vials with modified Karnovsky fixative solution (2.5% glutaraldehyde and 2% paraformaldehyde, buffered with 0.1M sodium cacodylate (pH 7.4) to be processed and histologically analyzed at CEME (Center for Electronic Microscopy) at UNIFESP/EPM, as follows: four consecutive baths in a buffer solution of sodium cacodylate (0.1M pH 7.4), being the two first baths at room temperature for 15 minutes; the third was done at 4º C for 12 hours; and the fourth at room temperature for 15 minutes. Post-fixation with 2% osmium tetroxide in buffer solution of sodium cacodylate (0.1 M pH 7.4) for one hour, followed by two quick successive baths (1 minute) in distilled water. Fragments were then dipped in aqueous solution of 0.5% uranyl acetate for 30 minutes, followed by two quick successive baths (1 minute) in distilled water. Dehydration followed, in the form of successive immersion baths in solutions with increasing ethanol contents: 70% for 30 minutes (once), 90% for 30 minutes (once) and 100% for 20 minutes (twice). Two consecutive immersion baths in propylene oxide for 20 minutes each. Injection into an Araldite 502® resin mix type: propylene oxide in concentrations of 1:3, 1:2 and 1:1 for 60 minutes each, agitated by rotation at room temperature. Placement into pure resin for four to five hours in vacuum. Transference to embedding molds horizontally oriented to allow cross-sectioning of the nerves followed by resin polymerization in oven at 60º C for 72 hours. The resulting blocks were trimmed and sectioned using a Leica Reichert Ultracuts® ultramicrotome. The blocks obtained after this preparation were trimmed and sectioned using an ultramicrotome equipped with glass blades, producing ultrafine 0.3μm cuts in thickness. Then the cuts were placed in glass slides and stained with 1% toluidine blue.
The cross sections were seen under a Nikon Optiphot II® light microscope coupled to a Leica Quantimet 500 QWIN® image analyzer system, having the images digitalized by a Sony XC 003P® camera. We obtained images with a 40x magnification lens and total magnification of 860 x the digitalized image. These images were then printed on 10 x 15cm photographic paper for the histometric quantitative and qualitative analysis of neural regeneration by analyzing myelinated fibers.
For the qualitative analysis, the pictures were displayed sequentially according to the time between the lesion and the animal slaughtering, and the presence of myelinated fibers was appreciated, together with its relative diameter and its uniform distribution in the nerve.
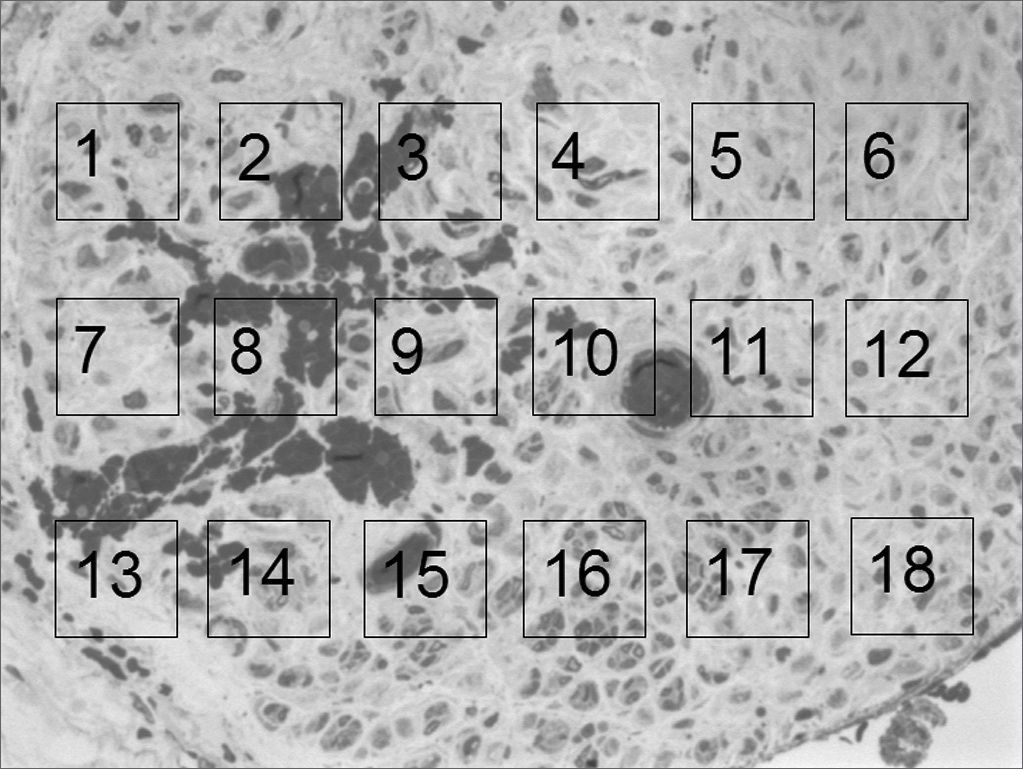
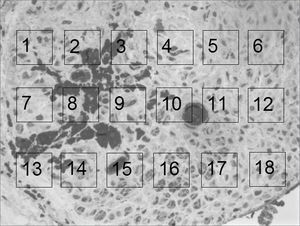
For the quantitative analysis we prepared a 10 x 15cm “mask” on a transparent acetate sheet, in which we drew 18 1.5 x 1.5cm “windows”, numbered from 1 to 18, uniformly distributed on 3 lines (6 “windows” per line). We used Figure 4, which will be shown in the results in order to carry out a photo assembling that will represent these “windows”. We then, randomly selected 3 “windows” per line in order to count myelinated fibers, at a total of 9 “windows” per image. Such count followed the classical hystometric method11 that only considers as “valid” those elements present totally “within” the window area and the ones only partially inside, but were crossed by the upper and left corners. We disregarded those that were partially in, and were crossed by the lower and right corners. The counts of each one of these nine “windows” was added up, thus yielding the total count for each rat. During the counting process, if any of the “windows” was considered inadequate, either because it was not totally inserted within the neural area (encompassing sheath and/or adjacent tissues), or the fibers be diagonally cut, or still because of problems as to its color (homogeneous deposits of toluidine blue). The “window” at hand here is then replaced by the next one in line. In case this new “window” is already selected at the initial random choosing, the succeeding window was then chosen. In case it becomes necessary to replace Windows 6, 12 or18 (last in the lines); we chose windows 1, 7 and 13 (first ones in the lines).
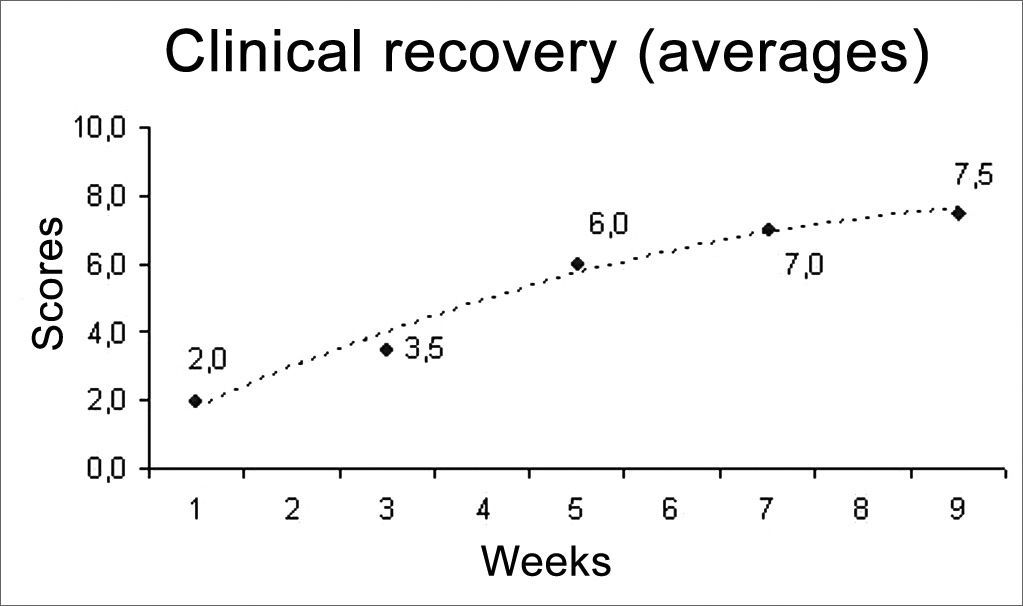
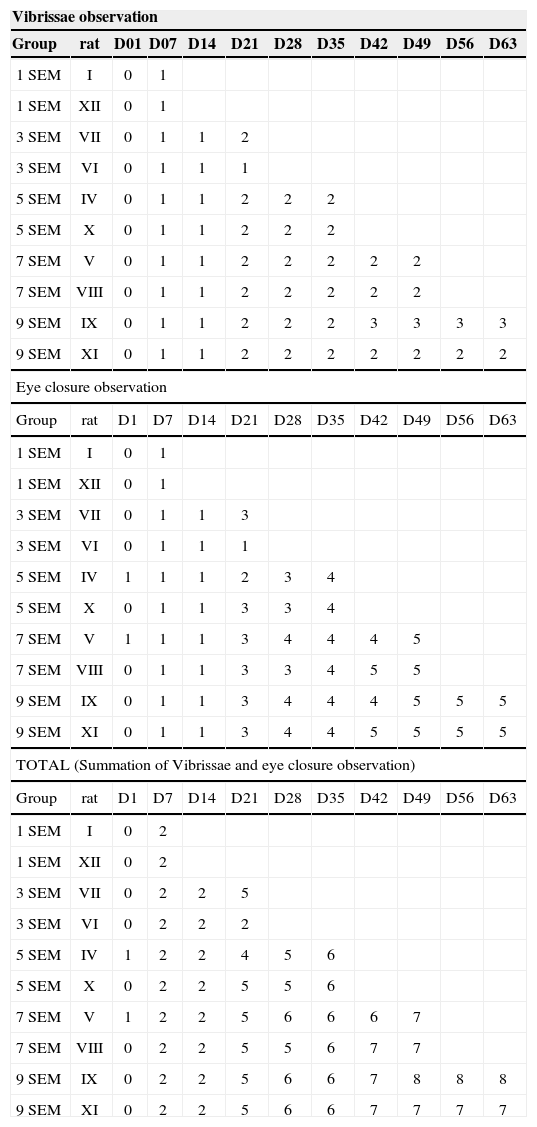
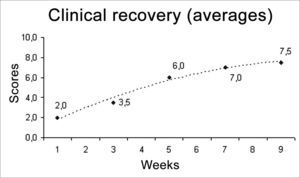
RESULTSWe used two animals in each study group, a total of 12 rats. The results from this behavioral observation are depicted on Table 1. The score obtained by each rat in relation to vibrissae movement and eye closure were added up and we used the average value from the animals from each group, depicted on Chart 1. In this one we may infer a temporal recovery in the animals along these nine weeks of clinical observation. Statistical analysis were not carried out, since we have a sample of only two animals per group; however, we see a clear trend towards clinical recovery, going from an average of 2 points in the 1SEM group, towards 7.5 points in the 9SEM group. A “trend line” was calculated and inserted in order to facilitate the perception of such fact.
Results of animal behavior observation.
| Vibrissae observation | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | rat | D01 | D07 | D14 | D21 | D28 | D35 | D42 | D49 | D56 | D63 |
| 1 SEM | I | 0 | 1 | ||||||||
| 1 SEM | XII | 0 | 1 | ||||||||
| 3 SEM | VII | 0 | 1 | 1 | 2 | ||||||
| 3 SEM | VI | 0 | 1 | 1 | 1 | ||||||
| 5 SEM | IV | 0 | 1 | 1 | 2 | 2 | 2 | ||||
| 5 SEM | X | 0 | 1 | 1 | 2 | 2 | 2 | ||||
| 7 SEM | V | 0 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | ||
| 7 SEM | VIII | 0 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | ||
| 9 SEM | IX | 0 | 1 | 1 | 2 | 2 | 2 | 3 | 3 | 3 | 3 |
| 9 SEM | XI | 0 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Eye closure observation | |||||||||||
| Group | rat | D1 | D7 | D14 | D21 | D28 | D35 | D42 | D49 | D56 | D63 |
| 1 SEM | I | 0 | 1 | ||||||||
| 1 SEM | XII | 0 | 1 | ||||||||
| 3 SEM | VII | 0 | 1 | 1 | 3 | ||||||
| 3 SEM | VI | 0 | 1 | 1 | 1 | ||||||
| 5 SEM | IV | 1 | 1 | 1 | 2 | 3 | 4 | ||||
| 5 SEM | X | 0 | 1 | 1 | 3 | 3 | 4 | ||||
| 7 SEM | V | 1 | 1 | 1 | 3 | 4 | 4 | 4 | 5 | ||
| 7 SEM | VIII | 0 | 1 | 1 | 3 | 3 | 4 | 5 | 5 | ||
| 9 SEM | IX | 0 | 1 | 1 | 3 | 4 | 4 | 4 | 5 | 5 | 5 |
| 9 SEM | XI | 0 | 1 | 1 | 3 | 4 | 4 | 5 | 5 | 5 | 5 |
| TOTAL (Summation of Vibrissae and eye closure observation) | |||||||||||
| Group | rat | D1 | D7 | D14 | D21 | D28 | D35 | D42 | D49 | D56 | D63 |
| 1 SEM | I | 0 | 2 | ||||||||
| 1 SEM | XII | 0 | 2 | ||||||||
| 3 SEM | VII | 0 | 2 | 2 | 5 | ||||||
| 3 SEM | VI | 0 | 2 | 2 | 2 | ||||||
| 5 SEM | IV | 1 | 2 | 2 | 4 | 5 | 6 | ||||
| 5 SEM | X | 0 | 2 | 2 | 5 | 5 | 6 | ||||
| 7 SEM | V | 1 | 2 | 2 | 5 | 6 | 6 | 6 | 7 | ||
| 7 SEM | VIII | 0 | 2 | 2 | 5 | 5 | 6 | 7 | 7 | ||
| 9 SEM | IX | 0 | 2 | 2 | 5 | 6 | 6 | 7 | 8 | 8 | 8 |
| 9 SEM | XI | 0 | 2 | 2 | 5 | 6 | 6 | 7 | 7 | 7 | 7 |
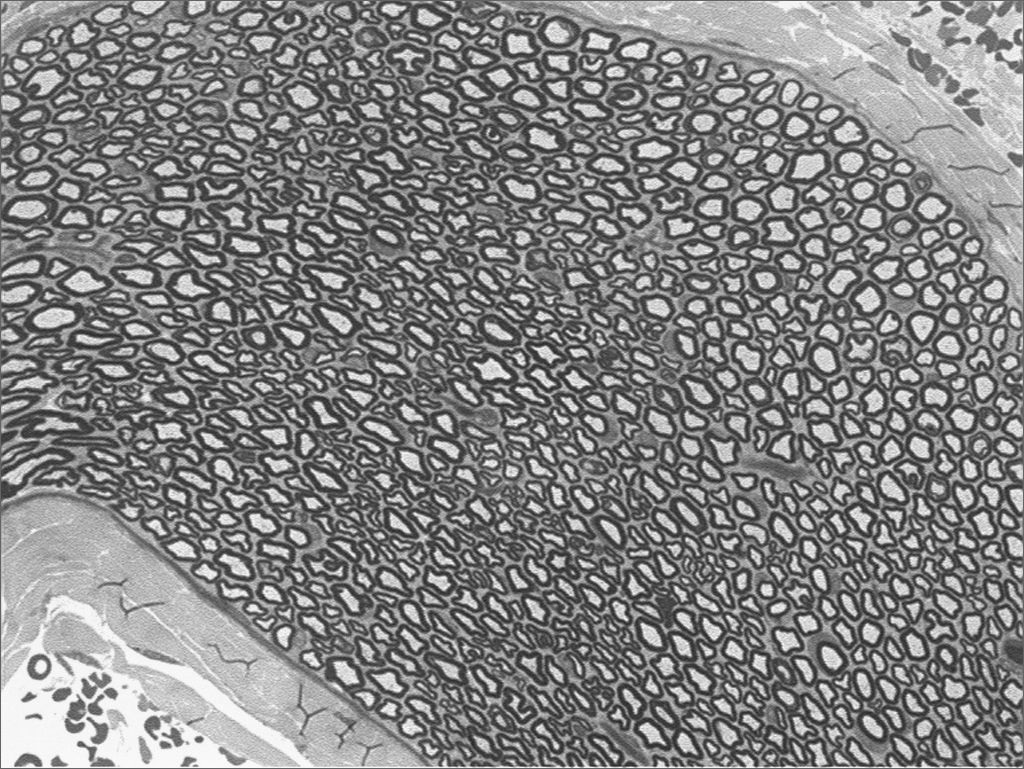
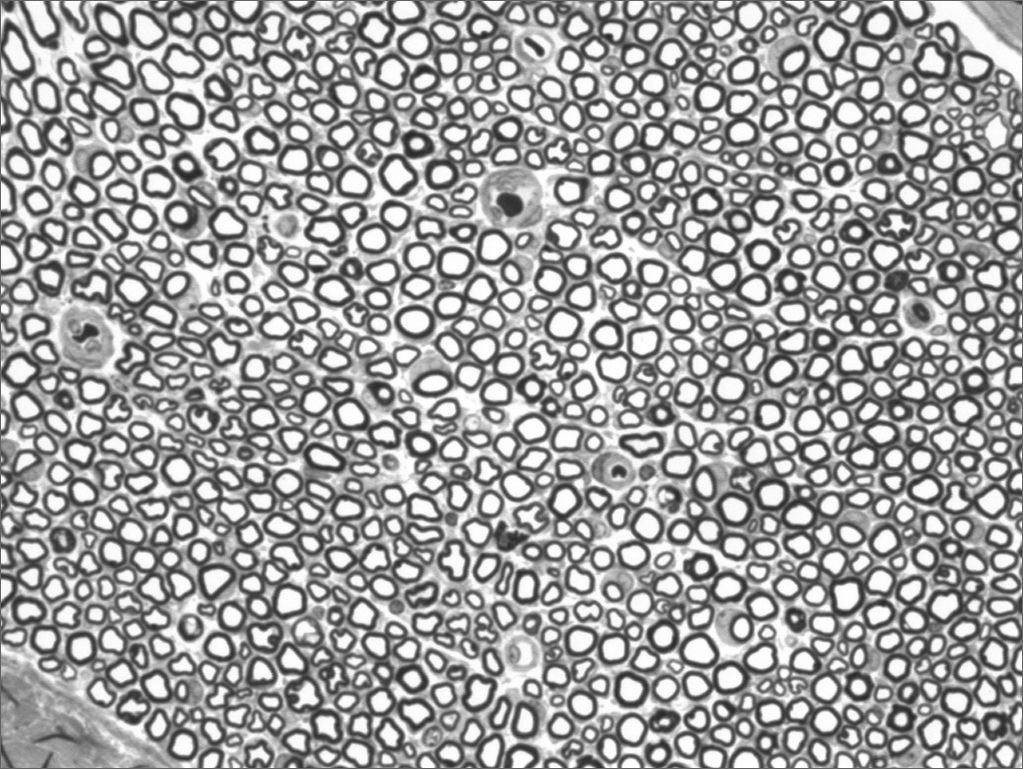
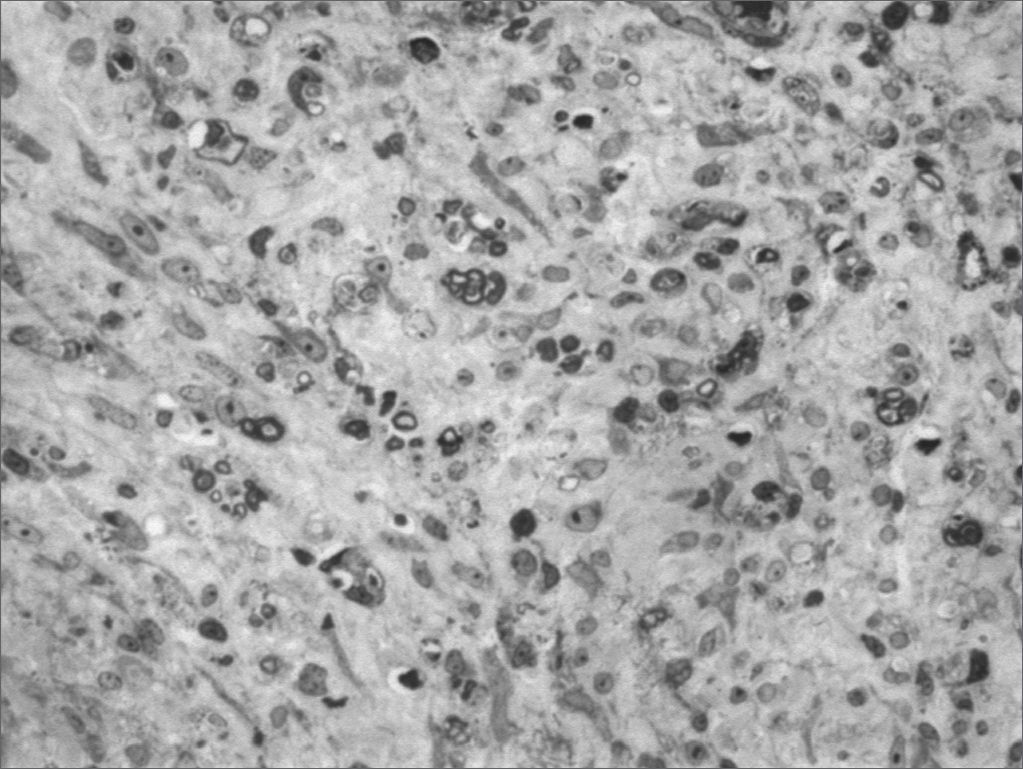
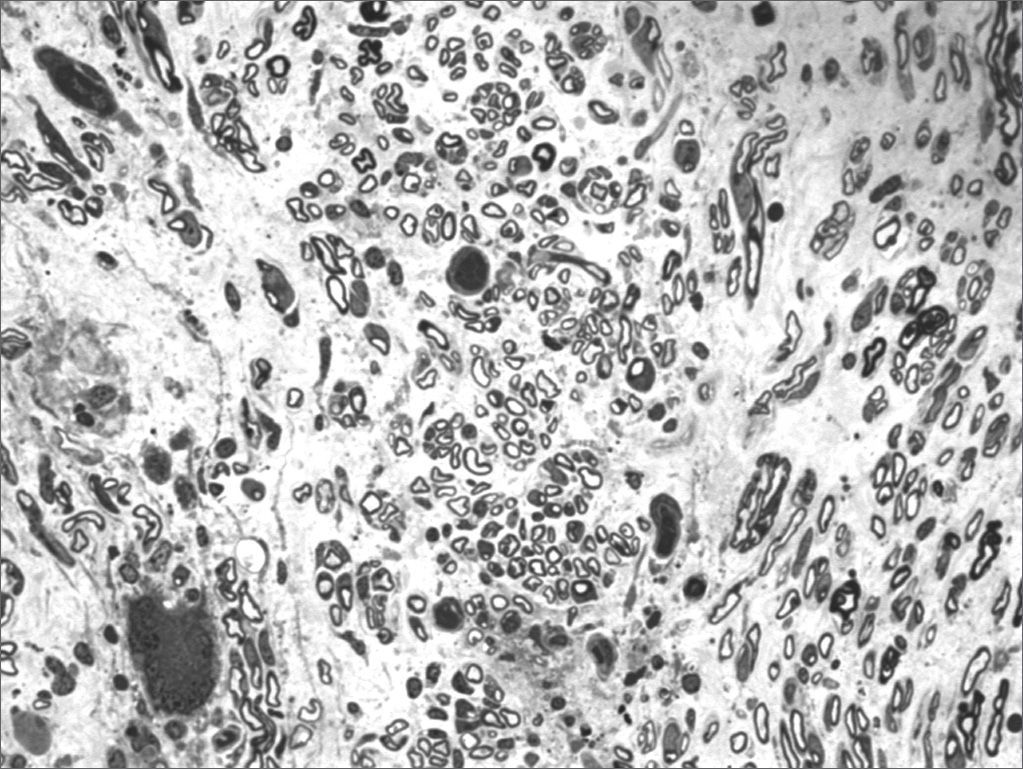
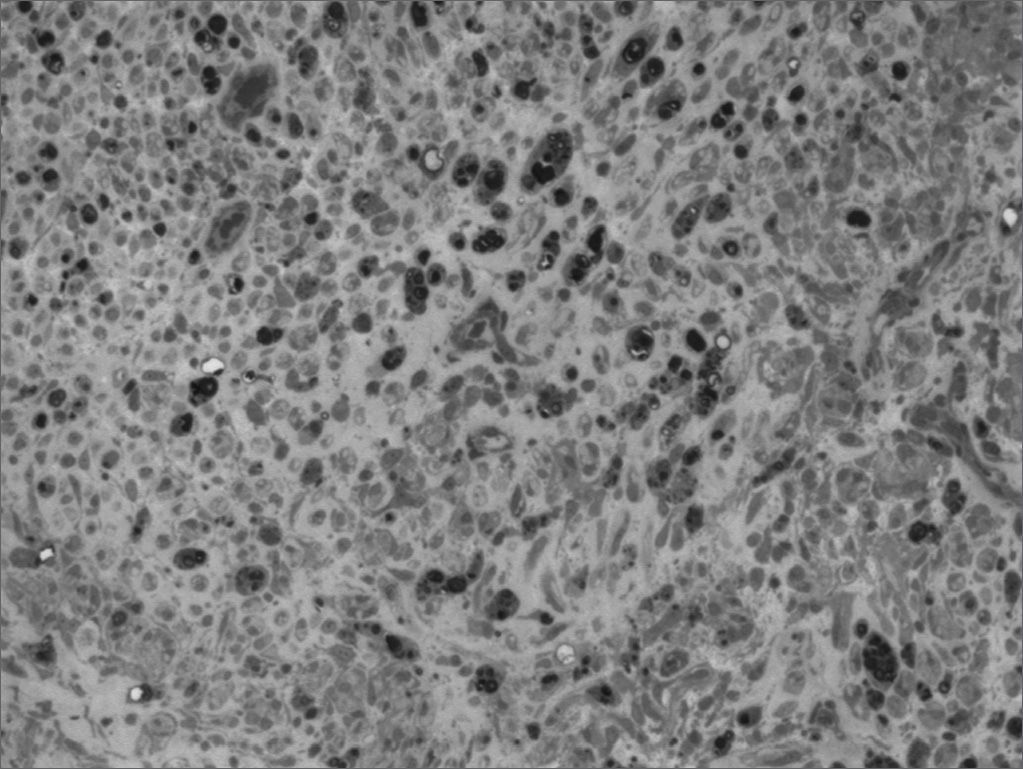
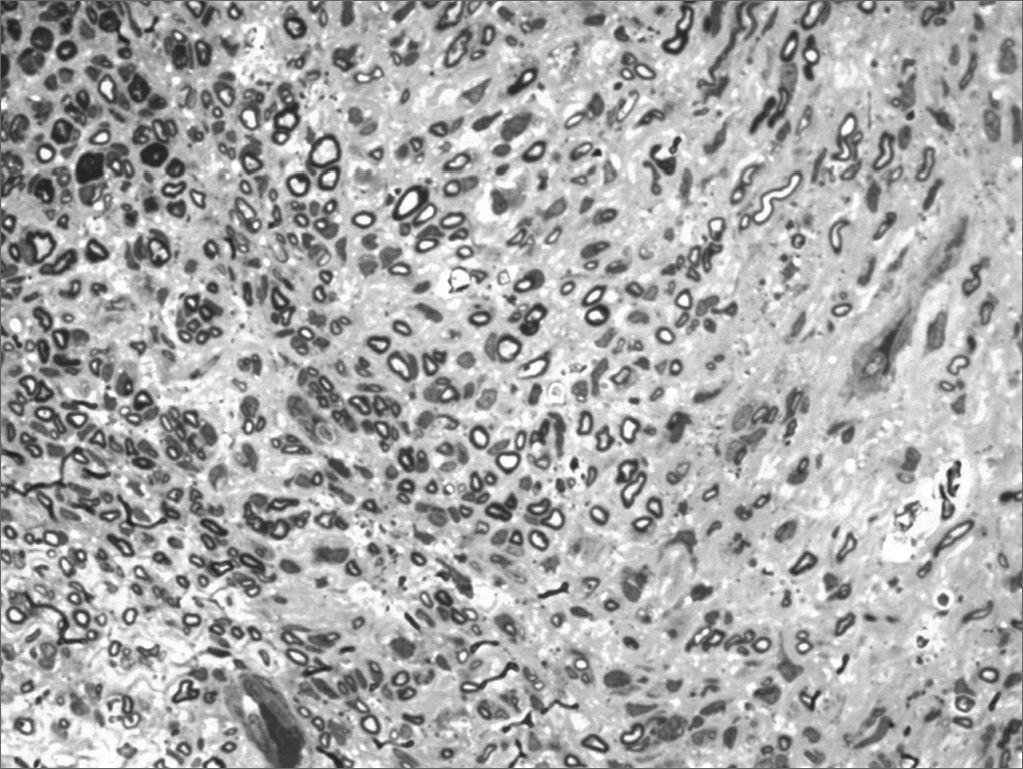
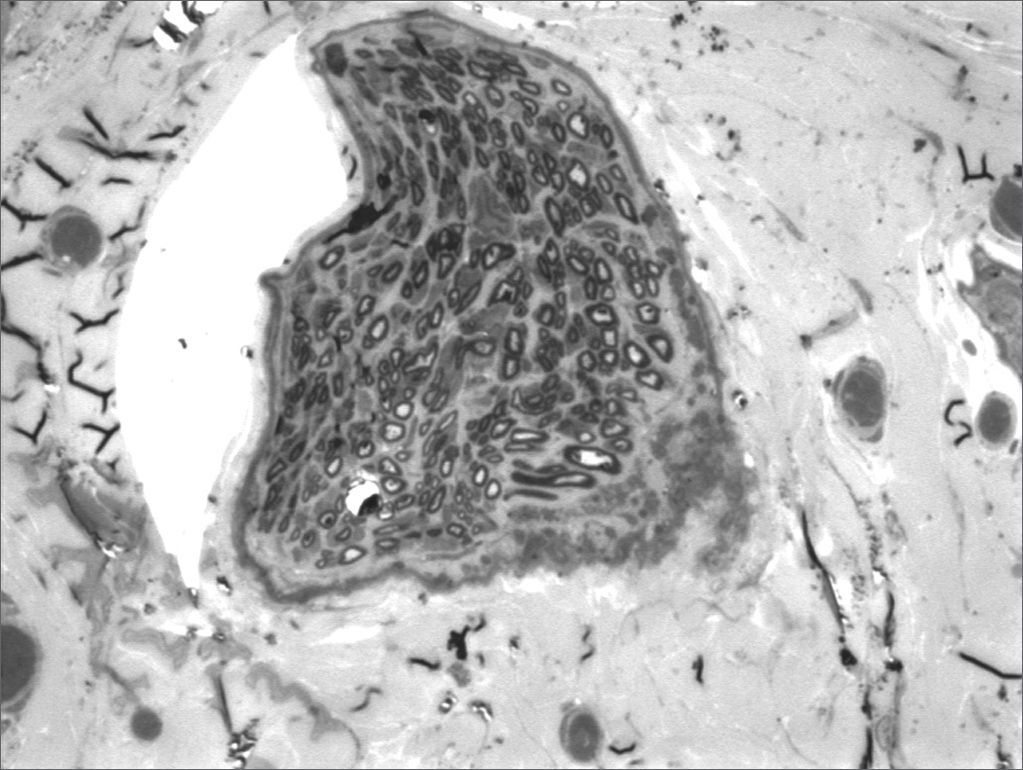
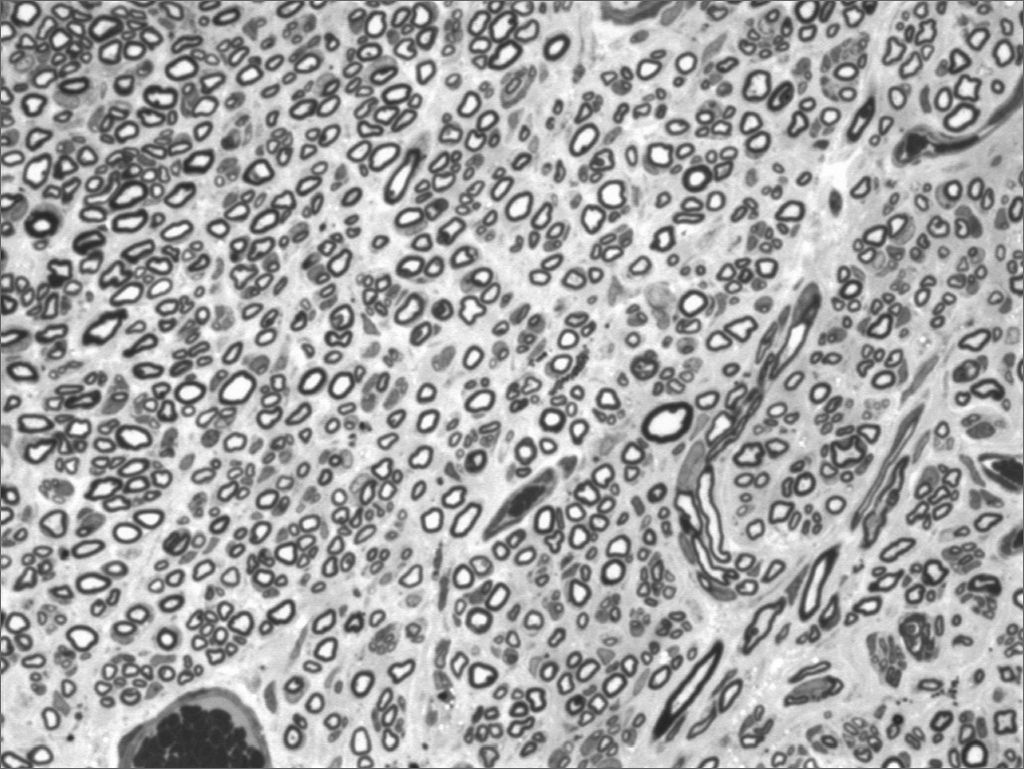
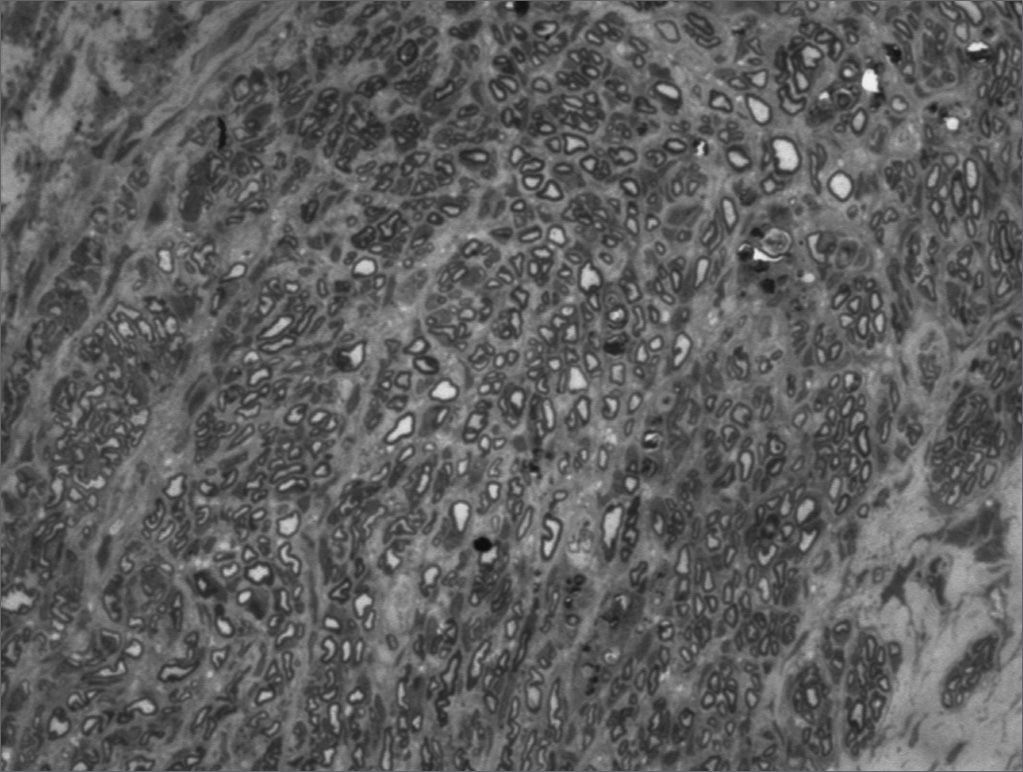
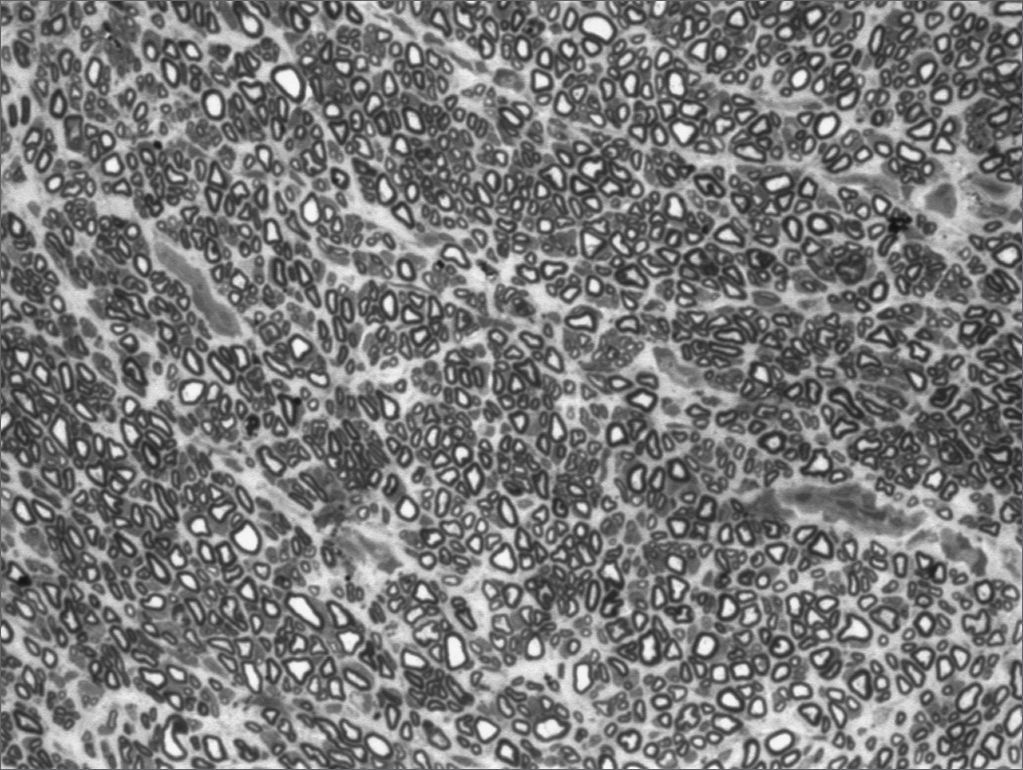
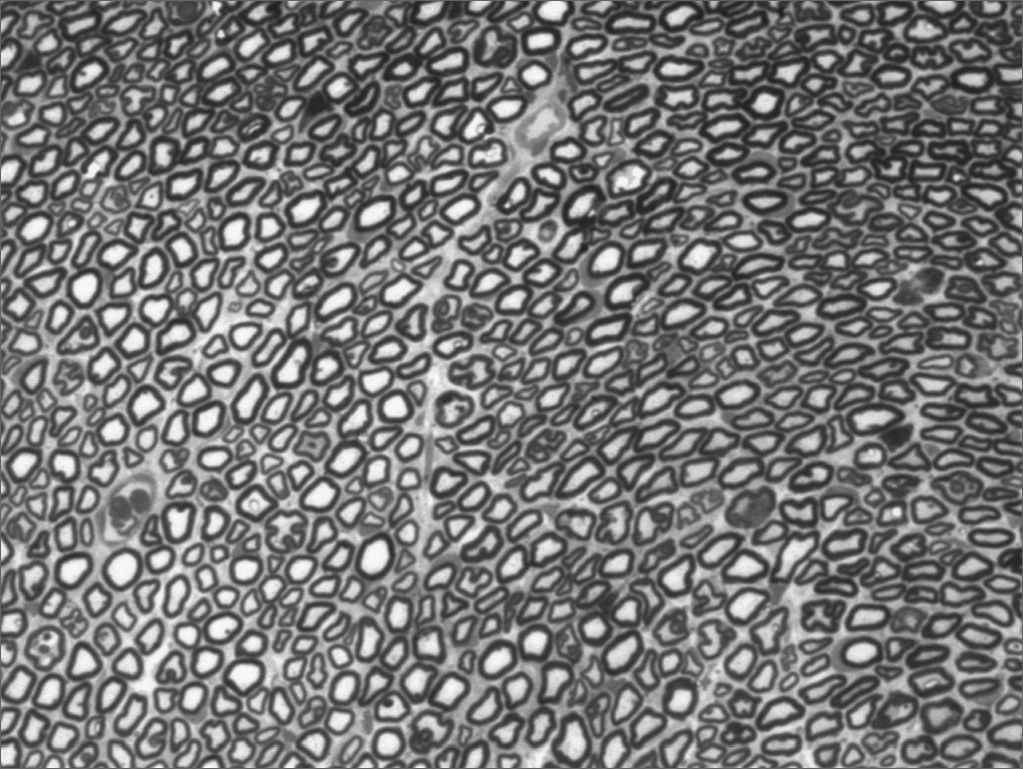
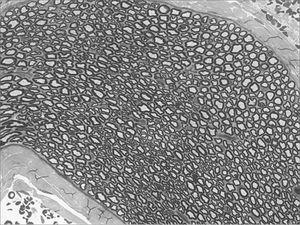
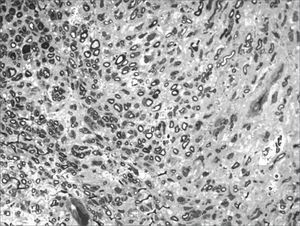
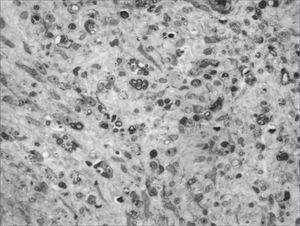
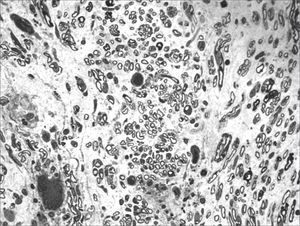
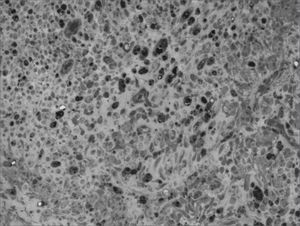
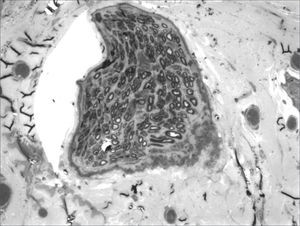
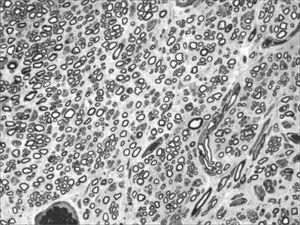
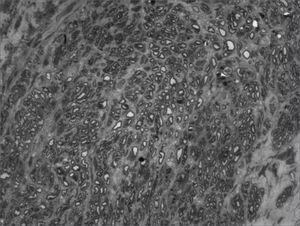
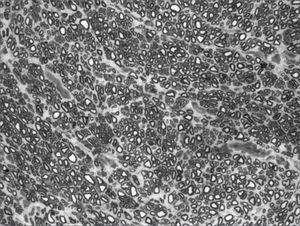
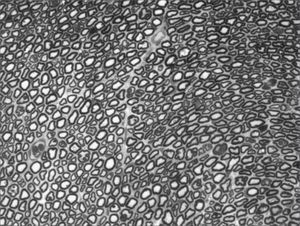
We, hereby show 12 pictures obtained from each one of the animals (Figures 1 through 12). By using these images in the qualitative histological analysis we can notice in the 0 SEM group (Figures 1 and 2) the presence of regular and circular fibers, homogenously distributed throughout the whole field. In the 1 SEM (Figures 3 and 4) group we hardly noticed any myelinated fiber, and in Figure 4 we see a dye build up in part of the field. In the 3 SEM group, observing Figure 5 (rat VII), we noticed the presence of small gauge myelinated fibers grouped around some nerve areas; now, on Figure 6 (rat VI), we noticed an image very much similar to what was seen in the 1 SEM group. In Figure 8 (rat X), from the 5 SEM group, we noticed a “retraction” of the material, and this prevented a qualitative analysis; however, on Figure 7, we observed an increase in the presence of small myelinated fibers throughout the whole neural area. In the 7 SEM (Figures 9 and 10) and 9 SEM (Figures 11 and 12) groups, such presence increases even further, already showing some larger fibers, similar to what we noticed for the 0 SEM group, especially in Figure 12 (rat IX).
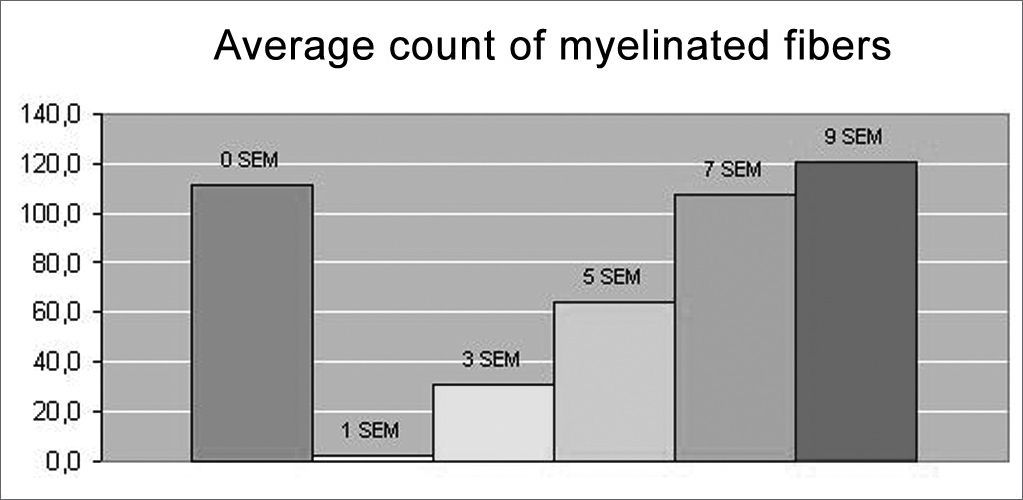
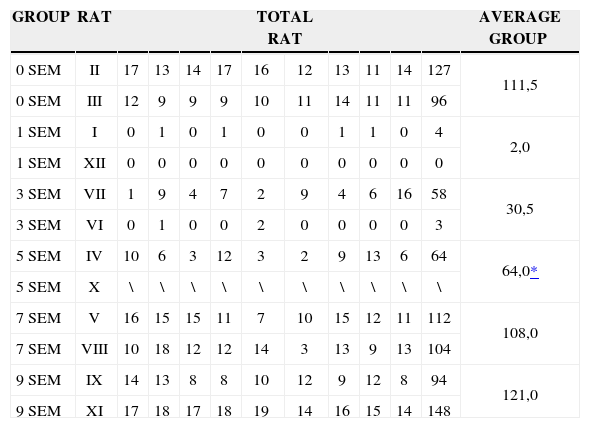
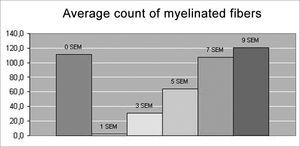
In the quantitative histology analysis, we had problems in two images. In Figure 4, because of the dye build up that was previously mentioned, it was necessary to change three of the nine windows randomly chosen in order to proceed with the count. In Figure 8, the material “retraction” made it impossible to carry out even its quantitative analysis. Table 2 depicts the histometric analysis data, compiling the counts of each one of the nine “windows” randomly chosen for each rat, total count for each animal and the average for each group. We noticed a temporal increment in the number of myelinated fibers, and for group 7 SEM we attained values that were very close to those from group 0 SEM, and this was surpassed in group 9 SEM. Chart 2 depicts this very fact. We also did not do statistical analysis here because we had a sample of only two animals per group.
Results of myelinated fibers count and average per group.
| GROUP | RAT | TOTAL RAT | AVERAGE GROUP | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 SEM | II | 17 | 13 | 14 | 17 | 16 | 12 | 13 | 11 | 14 | 127 | 111,5 |
| 0 SEM | III | 12 | 9 | 9 | 9 | 10 | 11 | 14 | 11 | 11 | 96 | |
| 1 SEM | I | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 4 | 2,0 |
| 1 SEM | XII | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 3 SEM | VII | 1 | 9 | 4 | 7 | 2 | 9 | 4 | 6 | 16 | 58 | 30,5 |
| 3 SEM | VI | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 3 | |
| 5 SEM | IV | 10 | 6 | 3 | 12 | 3 | 2 | 9 | 13 | 6 | 64 | 64,0* |
| 5 SEM | X | \ | \ | \ | \ | \ | \ | \ | \ | \ | \ | |
| 7 SEM | V | 16 | 15 | 15 | 11 | 7 | 10 | 15 | 12 | 11 | 112 | 108,0 |
| 7 SEM | VIII | 10 | 18 | 12 | 12 | 14 | 3 | 13 | 9 | 13 | 104 | |
| 9 SEM | IX | 14 | 13 | 8 | 8 | 10 | 12 | 9 | 12 | 8 | 94 | 121,0 |
| 9 SEM | XI | 17 | 18 | 17 | 18 | 19 | 14 | 16 | 15 | 14 | 148 | |
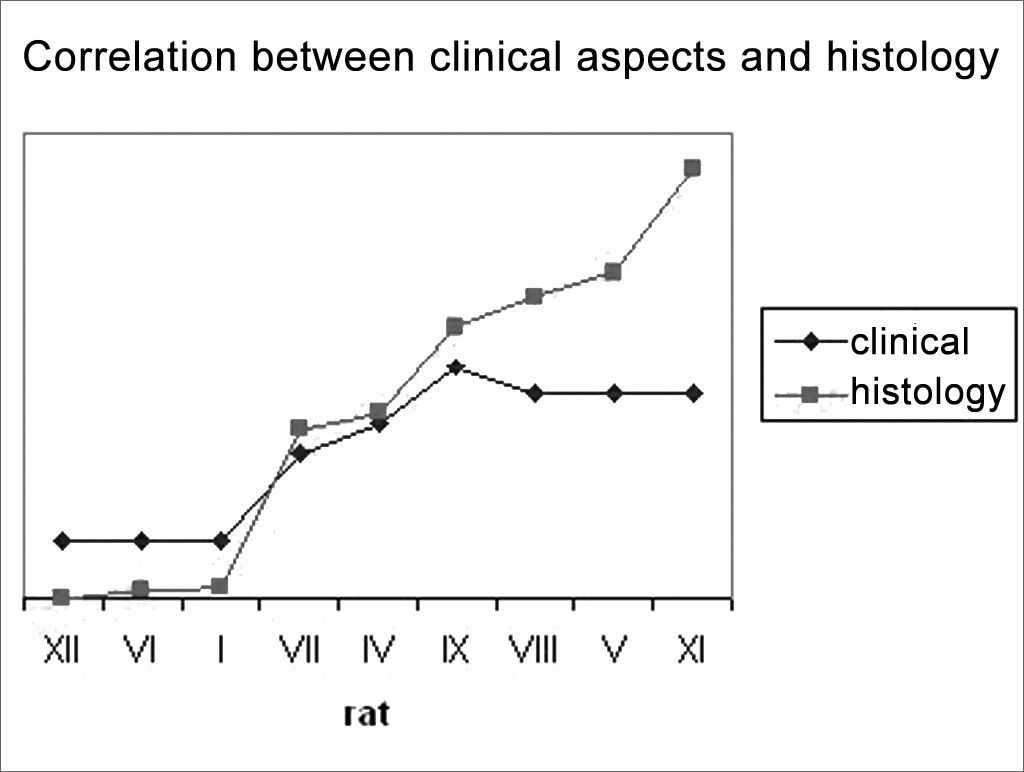
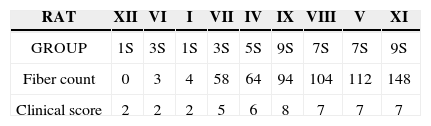
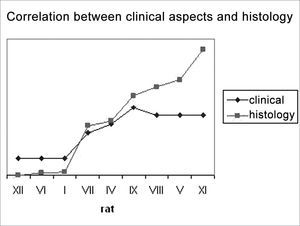
In an attempt to observe the correlation between the results obtained from clinical observation and the count of myelinated fibers, presented on Table 3 and Chart 3, that correlate these two series of results, we organized the rats in an ascending series of results in the counts of myelinated fibers, excluding rat X from this analysis, since we were unable to obtain a histometric analysis for it, as previously mentioned. Afterwards, we listed its results in the clinical observations and noticed a direct correlation between both results, especially in groups 1 SEM, 3 SEM and 5 SEM, in which a greater fiber count indicated a higher clinical score. Now, in groups 7 SEM and 9 SEM, a higher count did not point towards a better and directly related clinical result.
Setting up an experimental paradigm for a FN lesion is the first and fundamental step towards the study of actions from potential neurotrophic factors. Although we agree that we still lack a perfect experimental model12, such should bear at least the characteristics of being practical and easily reproducible, thus allowing for a temporal and objective analysis of the neural regenerative process. Many animals can be used in order to set up experimental models for the study of the FN, such as cats2, rabbits13, hamsters14, mice15, guinea pigs16 and pigs17. We chose the Wistar rat, because besides being an animal already used in similar studies published in the literature18–25, it is easily accessible in our Institution, it is easy to handle, it may be kept in collective cages, it is easily anesthetized and the animal with facial paralysis does not present greater morbidity in order to feed itself, even when the paralysis is bilateral12. We chose to standardize the model with male animals because there are reports on gender influence on neural regeneration, being naturally greater for female hamsters14, and also so that we would not have to control the animal hormonal cycle. The extratemporal facial nerve anatomy in rats is also described in the literature, its trunk has approximately 6mm, it is easy and constantly accessed by a simple behind-the-ear incision, bearing variations in less than 2.1% in serial counts of myelinated fibers, and it is not influenced by the animal age and weight12,26. As to the cons related to such animal, we noticed that the FN trunk is monofascicular, which is different from that of human beings12, besides the little facial expression in these animals, and this makes it difficult to establish visual scales of mobility recovery.
Once decided upon the animal, we have to choose the type of neural lesion. Numerous studies have been using the “crushing” of the FN8,21,27,28, however, we chose to section the nerve completely since it is a more reproducible lesion, always grade V in the Sutherland classification17,18. In repairing a complete FN lesion we could decide to use silicon tubes, collagen rods, fibrin glue, and temporal fascia or free neural or muscle grafts1,2,7,13. We also chose to do epineural suturing, because besides being well established in the literature12,16,18,19,20,24,25,29,30, it better mimics the surgical correction carried out in human being nerves31, and it was relatively easy to obtain a proper alignment and low stump tension with a single stitch as is the case in rats.1,7.
Numerous methods have been already proposed aiming at improving neural regeneration in animals. Behavioral scales8,28,32,33, electrophysiological analysis2,10,19,32, muscle fiber analysis19,20,29, central nuclei analysis18,19,21–23,30,34–36 and axon analysis2,10,16,19,31–33 have been described in the literature. We decided to use a behavioral analysis because it reflects not only an anatomical aspect, but also the main regeneration issue, which is facial movement function recover37. By means of FN lesion studies in rodents, other authors have been using clinical scales observing the movement of vibrissae, very similar to our own.8,28 In an attempt to enhance behavioral analysis; we associated eye closure observation, based on the experience of other authors who worked with other animals, such as cats2. Notwithstanding, it is undeniable that such behavioral measure has an important component of subjectiveness, and is less reproducible. We then decided to associate a histometric assessment by light microscopy of the axon myelinated fibers that besides reported in the literature, bears an acceptable cost16, 19, 31, 32, 37–39. It is important to stress some of the limitations of this technique. It does not allow to check the presence of non-myelinated fibers - only seen by means of electron microscopy, and such technique is of very high cost to be used in large series13,37 - and does not assess the diameter, nor the thickness of the myelinated sheath - items that would be interesting to be used in order to follow up the regeneration process. We tried to assess these last two items through automated analysis of areas captured by color matrix systems; however, the data were not reproducible and reliable, since there is great difficulty in dyeing myelin with a specific color - problem already reported by other authors37.
Once the model is established, we then discuss our results compared to the observations of other authors in the literature. As to the behavioral observation scale, we attained results that showed a temporal recovery of facial movement, well correlated to the results attained by other authors who used similar models with rodents27,28. Within a maximum score of 10, our animals had a recover of 50% in function between the third and the fifth weeks, and of 75% in the 9th week, when we terminated the experiment. Eye closure seemed to be the earliest and more easily observable item for this analysis, and vibrissae movement return was the slowest one. Such observation is in tune with that of other authors who stress that the blink reflex partial return is the earliest sign of neural recovery in rodents28.
As to our histology qualitative analysis, we observed the appearance of small groups of myelinated fibers, especially after the third week, and such process was intensified on the subsequent weeks, followed by an increase in their diameters, especially during the 7th and the 9th weeks. This observation finds corresponding ones in the literature in investigations carried out with rats, the FN and the sciatic nerve38–40, in which the authors show a gradual increase in the number and diameter of myelinated fibers alongside neural regeneration. This would reflect the initial multiple sprouting process of fibers from the proximal stump that invade the distal stump and that increase in diameter with time, thus reflecting a greater capacity for neural conduction. The time in which we started to notice neural regeneration is also in agreement with the classical observations already described in the literature, in which Wallerian Degeneration would be completed around the 2nd week; and fiber sprouting from the proximal stump fibers and the onset of distal stump “invasion” would happen around the 10th day1,7,41.
As to our histometric quantitative analysis, we observed that after the 3rd week there was a gradual increase in myelinated fibers count, reaching a value very close to normal on the 7th week, and surpassing that on the 9th week. Such fact also finds similarities in the literature, where many authors report a progressive increase on the number of myelinated fibers alongside the regeneration process38–40. In our opinion, the very fact that the 9th week count surpasses that of the control group (OSEM), is due to the smaller fiber diameter in this group when compared to those of the control group, such fibers would be more “immature” than those of greater diameter. So much so, that the IX-9SEM (Figure 9) rat was the one that presented the highest qualitative similarity of fibers in comparison to group O SEM, and also a better clinical score, had less fibers than the XI-9SEM, V-7SEM and VIII-7SEM rats. However, since we did only 2 animals per group, it would not be adequate to make any statistical comparison among them.
When we compare the results of our clinical and histometric clinical behavior analysis (Chart 3), we can see that a higher clinical score is followed by a greater fiber count, especially up to a plateau made up by the animals in groups 7SEM and 9SEM, where the count remains ascending and the clinical improvement is practically stable. Such later dissociation among neural regeneration parameters that occurred in the last weeks seems to happen because of two facts. First, our clinical evaluation scale is somewhat limited, since we highlighted previously that rats do not have refined facial movements available for such. And second, that an increase in the number of fibers only, does not necessarily reflect an improvement in final movement function, and such fact has also been stressed in the literature1,7,37,42.
CONCLUSIONWe conclude that there is no perfect experimental paradigm for the study of FN recovery after a lesion. Notwithstanding, we believe to have established a proper method through behavioral and histometric analysis.
Paper submitted to the ABORL-CCF SGP (Management Publications System) on May 2, 2006 and accepted for publication on June 20, 2006. cod. 1891.
- Home
- All contents
- Publish your article
- About the journal
- Metrics