The biological processes involved in noise-induced hearing loss (NIHL) are still unclear. The involvement of inflammation in this condition has been suggested.
ObjectiveTo investigate the association between interleukin – 6 (IL-6) polymorphism and susceptibility to NIHL.
MethodsThis was a cross-sectional study with a sample of 191 independent elderly individuals aged >60 years of age. Information on exposure to occupational noise was obtained by interviews. Audiological evaluation was performed using pure tone audiometry and genotyped through PCR by restriction fragment length polymorphism – PCR-RFLP. Data were analyzed using the chi-square test and the odds ratio (OR), with the significance level set at 5%.
ResultsAmong elderly with hearing loss (78.0%), 18.8% had a history of exposure to occupational noise. There was a statistically significant association between the genotype frequencies of the IL-6 −174 and NIHL. The elderly with the CC genotype were less likely to have hearing loss due to occupational noise exposure when compared to those carrying the GG genotype (OR=0.0124; 95% CI 0.0023–0.0671; p<0.001).
ConclusionThis study suggests there is an association of polymorphisms in the IL-6 gene at position – G174C with susceptibility to noise-induced hearing loss.
Os processos biológicos envolvidos na perda auditiva induzida por ruído (PAIR) ainda não estão claros. O envolvimento de processo inflamatório nesta condição tem sido sugerido.
ObjetivoInvestigar a associação entre o polimorfismo no gene da interleucina-6 (IL-6) e a suscetibilidade à PAIR.
MétodoTrata-se de estudo transversal com amostra de 191 idosos independentes acima de 60 anos de idade. Informações sobre a exposição ao ruído ocupacional foram obtidas por entrevistas. A avaliação audiológica foi realizada por meio de audiometria tonal liminar e a genotipagem pela técnica da PCR-RFLP. Os dados foram analisados usando-se o teste Qui-quadrado e a razão de chances (OR), com o nível de significância fixado em 5%.
ResultadosEntre os idosos com perda auditiva (78,0%), 18,8% apresentavam histórico de exposição ao ruído ocupacional. Houve associação estatisticamente significante entre as frequências genotípicas da IL-6 -174 e a PAIR. Os idosos portadores do genótipo CC foram menos propensos a apresentar perda auditiva por exposição ao ruído ocupacional quando comparados a aqueles portadores do genótipo GG (OR=0,0124; 95% IC 0,0023-0,0671; p<0,001).
ConclusãoO presente estudo sugere a associação do polimorfismo no gene da IL-6 na posição -G174C com a suscetibilidade à perda auditiva induzida por ruído.
Elderly health has increasingly aroused the interest of researchers, as the population in both developed and developing countries is aging.1 It is estimated that by 2040 developing countries will have a billion people aged 60 years or older.2 Given the rapidity and magnitude of this increase, care for this specific group is essential, so they can age in good health and with good quality of life.3
The most common types of hearing loss are presbycusis (due to aging) and noise-induced hearing loss (NIHL).4 NIHL is due to continued exposure to loud noise levels, resulting in gradual loss of auditory acuity and is usually bilateral, symmetric, sensorineural and irreversible. It usually affects the high audiometric frequencies of 3000–6000Hz.5 The etiology of hearing loss also has genetic components, in addition to environmental ones, and more recently, inner ear inflammatory response induction and up-regulation of proinflammatory cytokines were evaluated in the presence of exacerbated noise.5–7 Several researchers have reported the presence of inflammatory cells in the steady state and their increase after lesions in the inner ear.6,8–10
Noise exposure induces the expression of pro-inflammatory cytokines, including tumor necrosis factor-α (TNF-α), interleukin 1β (IL-1β) and interleukin-6 (IL-6).11 So et al.12 observed a transient up-regulation of IL-6 in cisplatin-treated animal models. Wakabayashi et al.13 investigated the effect of IL-6 inhibition using an anti-IL-6 (MR16-1) antibody in mice. These authors found that MR16-1 showed a protective effect against noise-induced cochlear injury, mainly due to neuronal loss suppression and presumably by relieving the inflammatory response, similar data were found by Nakamoto et al.14 These authors suggested that the suppression of proinflammatory cytokine HSF-1 in the cochlea by the administration of GGA (geranyl–geranyl–acetone) could be an important way to protect the inner ear.
The expression of cytokines may be influenced by genetic variation, resulting in pathogenic conditions15 and several studies have investigated single nucleotide polymorphisms (SNPs) as risk factors for inflammatory diseases.16 These SNPs may affect the expression, secretion and cellular transport of interleukins17,18 and can also decrease the level of the IL-1 receptor antagonist (IL-1Ra), which increases the production and activity of IL-1β.19
The SNP in the region −174 of the interleukin-6 gene contains the replacement of G by C, and the presence of the C allele represents a protective function due to reduced production of IL-6.20
Considering that NIHL is involved in the inflammatory process, this study aimed to evaluate the association between the proinflammatory cytokine-6 (IL-6) gene polymorphism and susceptibility to NIHL in physically independent elderly Brazilians.
MethodStudy populationThis cross-sectional study was approved by the Ethics Committee on Human Research of the University of Northern Paraná (0070/09). It is part of a broader investigation, the “Study on aging and longevity”, which has been carried out in Londrina since 2009. Londrina (approximately 500,000 inhabitants) is located in the northern region of the state of Paraná, Brazil.
Of a population of 43,610 elderly individuals treated in 38 basic health units (BHUs) in the urban area of the city, the sample size was set at 343 individuals, considering a confidence interval of 95% and a margin of error of 5%.21
Aiming at obtaining sample representativeness, random stratification was performed considering gender and regions of the city. The study included individuals aged 60 years or older, of both genders, living independently and classified at level 3 or 4, as proposed by Spirduso.22 This classification assesses the level of independence of the elderly, in which level 1 indicates a lack of self-mobility and level five indicates athletes. The elderly who had some disease or limitations that prevented the tests, such as physical or mental impairment, were excluded from the sample. All participants signed an informed consent form.
Audiological assessmentThe audiological assessment was performed by conventional pure tone audiometry, in an interacoustics audiometer. To determine the severity of hearing loss, the criteria proposed by BIAP – Bureau Internacional d’Audio Phonologie – an institution formed by several associations of European countries with the main objective of guiding the activity of professionals in these regions, were used. The means of hearing thresholds in dB in air conduction, at 500Hz, 1kHz, 2kHz and 4kHz and the 02/1 1997 recommendations were analyzed, both the right and left ears were evaluated for a group of individuals with a history of occupational noise and the group of individuals with no history of occupational noise.23–25
Evaluation of occupational noise exposureThe assessment of occupational noise exposure was performed through interviews with the elderly participants, using a semi-structured questionnaire. Information was collected on whether work was performed in a noisy environment or not, how many years of work in a noisy environment and whether the individual wore a hearing aid. Moreover, demographic characteristics (gender, age and ethnicity) were collected.
Genotyping for IL-6 G-174C (rs1800795) gene polymorphismPeripheral blood samples were collected in vacuum tubes containing 6% ethylenediamine tetraacetic acid (EDTA) and DNA was extracted from peripheral blood leukocytes, using the protocol described by Olerup and Zeterquist.26
Based on the original sample, the DNA was diluted to a concentration of 100ng/mL. The concentration was determined by spectrophotometer at 260nm and 280nm (Biomate 3, Thermo Fisher Scientific, Madison, USA).
The C to T polymorphic site located at position −174 of the IL-6 (rs1800795) gene was amplified by polymerase chain reaction (PCR) resulting in a 99bp fragment. The PCR mixture contained a mixture of a buffer solution pH 8.0 1×, 1.5mM of MgCl2, 8mM each of deoxyribonucleotide triphosphates, 1μM of each primer and 1U Taq DNA polymerase (Invitrogen, Carlsbad, CA, USA). The primers used were F: 5′-TTGTCAAGACATGCCAAGTGCT-3′ and R: 5′-GCCTCAGAGACATCTCCAGTCC-3′ (Invitrogen, Carlsbad, CA, USA).27
PCR amplification was performed in a thermocycler (TC-512 Techne resistance, Burlington, NJ, USA) under the following conditions: initial denaturation at 95°C for 5min followed by 30 cycles of 95°C for 1min, 56°C for 1min and 72°C for 1min, and final extension at 72°C for 5min.
The PCR product was digested with 2U of restriction enzyme NlaIII (Invitrogen, Carlsbad, CA, USA) overnight at 65°C. The digested fragments were separated on 2% agarose gel (Invitrogen Life Technologies, São Paulo, Brazil). A sample with known genotype was used as positive control and ultra-pure water was used as negative PCR control.
The 100-bp DNA molecular weight marker (Ladder, Invitrogen) was included on each gel stained with SybrSafe (Invitrogen Life Technologies, São Paulo, Brazil) and visualized under UV illumination. The reading and interpretation of agarose gels were made with LabImage L-PIX (HE) 1D-L340 (Loccus Biotechnology, São Paulo, Brazil) program. Fragments of 13, 227 and 59bp (allele G), and fragments of 13, 118, 109 and 59bp (allele C) were observed.
Statistical analysisThe statistical analysis was performed using the statistical package SPSS 17.0 (SPSS Inc., Chicago, IL, USA). To test the association between genotype frequency and hearing loss related to a history of occupational noise exposure, the chi-square test was performed. The Hardy–Weinberg equilibrium was tested in each group using the chi-square test. The odds ratio (OR) with a confidence interval of 95% (95% CI) was calculated. The significance level was set at 5%.
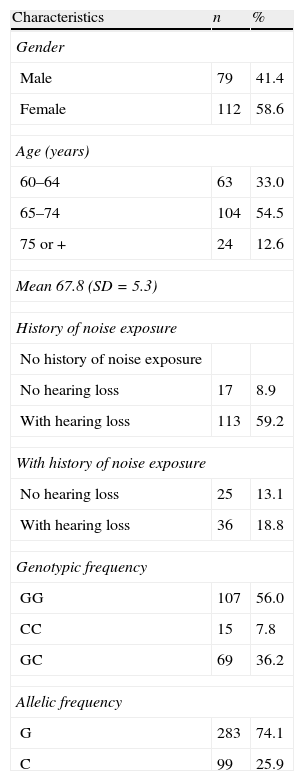
ResultsOf a total of 343, the molecular genetic procedures were performed in 191 elderly. Of these, 31.9% reported a history of noise exposure, with 18.8% exhibiting hearing impairment. Of the 68.1% with no history of noise exposure, 59.2% had hearing loss. The mean age was 67.8±5.3 years, with a higher proportion (58.6%) of elderly women (Table 1).
General characteristics, allelic and genotypic frequencies among Brazilian elderly (n=191).
| Characteristics | n | % |
| Gender | ||
| Male | 79 | 41.4 |
| Female | 112 | 58.6 |
| Age (years) | ||
| 60–64 | 63 | 33.0 |
| 65–74 | 104 | 54.5 |
| 75 or + | 24 | 12.6 |
| Mean 67.8 (SD=5.3) | ||
| History of noise exposure | ||
| No history of noise exposure | ||
| No hearing loss | 17 | 8.9 |
| With hearing loss | 113 | 59.2 |
| With history of noise exposure | ||
| No hearing loss | 25 | 13.1 |
| With hearing loss | 36 | 18.8 |
| Genotypic frequency | ||
| GG | 107 | 56.0 |
| CC | 15 | 7.8 |
| GC | 69 | 36.2 |
| Allelic frequency | ||
| G | 283 | 74.1 |
| C | 99 | 25.9 |
Of the assessed elderly individuals, 56% were homozygous for the G allele, 7.8% were homozygous for the C allele and 36.2% were heterozygous (Table 1). The genotype distribution for IL-6 gene was in agreement with the Hardy–Weinberg equilibrium (p>0.05).
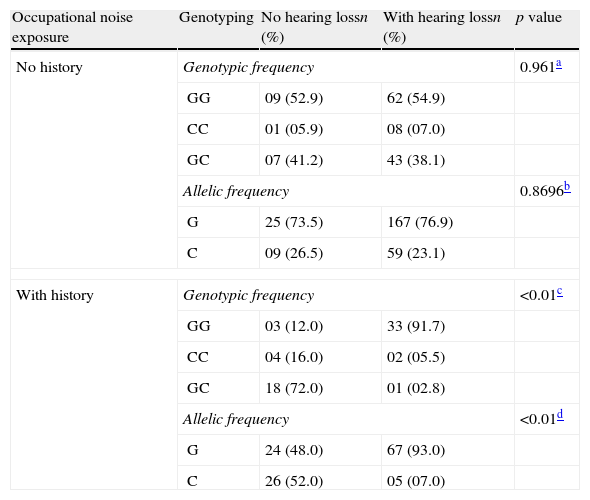
A statistically significant association was found between the genotype and allele frequencies of the IL-6 −174 (rs1800795) gene and hearing loss among elderly adults with a history of occupational noise exposure (p<0.001) (Table 2). The elderly with the CC genotype were less likely to have hearing loss due to occupational noise exposure in relation to the elderly with the GG genotype (OR=0.0124; 95% CI: 0.0023–0.0671; p<0.0001). However, this association was not observed in the elderly group without a history of noise exposure (χ2=0.078; p=0.961).
Association between genotype frequency for the interleukin-6 G-174C gene polymorphism and hearing loss related to history of occupational noise exposure (n=191).
| Occupational noise exposure | Genotyping | No hearing lossn (%) | With hearing lossn (%) | p value |
| No history | Genotypic frequency | 0.961a | ||
| GG | 09 (52.9) | 62 (54.9) | ||
| CC | 01 (05.9) | 08 (07.0) | ||
| GC | 07 (41.2) | 43 (38.1) | ||
| Allelic frequency | 0.8696b | |||
| G | 25 (73.5) | 167 (76.9) | ||
| C | 09 (26.5) | 59 (23.1) | ||
| With history | Genotypic frequency | <0.01c | ||
| GG | 03 (12.0) | 33 (91.7) | ||
| CC | 04 (16.0) | 02 (05.5) | ||
| GC | 18 (72.0) | 01 (02.8) | ||
| Allelic frequency | <0.01d | |||
| G | 24 (48.0) | 67 (93.0) | ||
| C | 26 (52.0) | 05 (07.0) | ||
Although the mechanism and role of proinflammatory cytokines in NIHL are not well understood, it is known that the structure and expression of cytokines may be affected by genetic variation, such as single nucleotide polymorphisms (SNPs), which results in evident pathological consequences.15 SNP functionality regarding gene expression is an important subject in disease-associated studies.16
In the present study, the frequencies of alleles G and C in the study population were 74.1% and 25.9%, respectively. Other studies have also demonstrated a predominance of the ancestral allele (G) ranging from 60% to 70%.28 Furthermore, we observed a higher frequency of GG genotype (56%), followed by GC (36.2%) and CC (7.8%). This is consistent with the aforementioned studies.20,28
We observed an association between the GG genotype of IL-6 and hearing loss with history of exposure to occupational noise (χ2=40.201; p<0.001). The elderly with the CC genotype had lower susceptibility to hearing loss due to occupational noise exposure (OR=0.0124; 95% CI: 0.0023–0.0671; p<0.01) compared to elderly patients with the GG genotype. The presence of the C allele results in lower expression of IL-6 after an inflammatory stimulus, when compared with the G allele.29 It is suggested that the CC genotype confers a protective effect against the development of comorbidities, which can be verified in this analysis, in which the frequency the C allele in the hearing loss group without exposure to noise was higher than in the NIHL group.
The studies by Hirose et al.6 and Fujioka et al.11 pointed out the possibility of inflammatory changes in the cochlea after noise stimuli. Fujioka et al.11 demonstrated for the first time the induction of pro-inflammatory cytokines in the cochlea exposed to the noise and observed increased expression of interleukins TNF-α, IL-1β and IL-6 3h after noise exposure. These authors pointed out that this is a self-protection mechanism against exposure to large amounts of noise and that the consequent over-expression of interleukins for long periods must worsen cochlear function.
The findings by So et al.12 clearly demonstrated that IL-6 acts as an inducer of acute cochlear damage, considering that a transient up-regulation of IL-6 occurred after exposure of animals with cisplatin. Some studies have shown that the inhibition of IL-6 production has a cochlear protective effect. Wakabayashi et al.21 used anti-IL-6 (MR10-1) antibody to inhibit IL-6 production in mice, identifying a protective effect for noise-induced cochlear injury. Along this same line of investigation, Nakamoto et al.14 found that the drug geranyl–geranyl–acetone (GGA) stimulates expression of the HSF gene which, in turn, inhibits inflammation in the cochlea, protecting the inner ear.
It is noteworthy that this strong evidence of the involvement of IL-6 with NIHL has been verified by several studies in animal models.11–14 Evidence in human beings related to pro-inflammatory interleukins and NIHL was recently reported by our group, when we investigated the association of polymorphisms in the IL-1β gene and NIHL.30 That study demonstrated that the polymorphism in this gene is not associated with NIHL in the assessed elderly subjects. However, the findings of the present study showed that polymorphisms in the IL-6 gene should contribute to the risk of NIHL in the Brazilian elderly.
Considering this context, the identification of the polymorphism in the IL-6 gene in patients with a history of hearing loss related to occupational exposure to noise may help us to understand individual variability of inflammation resulting in hearing loss, as well as, in the future, to suggest genotyping individuals for this particular polymorphism (rs1800795) in order to determine individual susceptibility, providing a new strategy for the prevention of hearing loss related to noise exposure.
ConclusionsAn association between the presence of IL-6 gene polymorphism and hearing loss associated with occupational noise exposure was found in elderly Brazilians. Studies based on large populations and with different ethnicities should be performed to confirm our findings.
FundingThis study was funded by FUNADESP / Brazil (Fundação Nacional de Desenvolvimento do Ensino Superior Particular).
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Braga MP, Maciel SM, Marchiori LL, Poli-Frederico RC. Association between interleukin-6 polymorphism in the −174 G/C region and hearing loss in the elderly with a history of occupational noise exposure. Braz J Otorhinolaryngol. 2014;80:373–8.
Institution: Universidade Norte do Paraná (UNOPAR), Londrina, PR, Brazil.