Saliva plays a key role in the homeostasis of the digestive tract, through its inorganic components and its protein growth factors. Sjögren's syndrome patients have a higher prevalence of gastroesophageal reflux disease and laryngopharyngeal reflux. Decreased salivary transforming growth factor alpha levels were observed in dyspeptic patients, but there have been no studies in patients with Sjögren's syndrome and laryngopharyngeal reflux.
ObjectiveTo compare the salivary transforming growth factor alpha levels of patients with Sjögren's syndrome and laryngopharyngeal reflux to those of healthy controls.
MethodsThis is a prospective controlled study. Twelve patients with Sjögren's syndrome and laryngopharyngeal reflux and 11 controls were prospectively evaluated. Spontaneous and stimulated saliva samples were obtained to establish salivary transforming growth factor alpha concentrations.
ResultsThe salivary transforming growth factor alpha levels of patients were significantly higher than those of healthy controls. Five patients with laryngopharyngeal reflux also had erosive esophagitis; their salivary transforming growth factor alpha levels were comparable to controls.
ConclusionSalivary transforming growth factor alpha level was significantly higher in patients with Sjögren's syndrome and laryngopharyngeal reflux when compared to the control group.
A saliva exerce influência primordial na homeostase do sistema digestório, pelos seus componentes inorgânicos e pelos fatores de crescimento. Indivíduos com síndrome de Sjögren (SS) apresentam maior incidência da doença do refluxo gastroesofágico (DRGE) e do refluxo laringofaríngeo (RLF). Concentrações salivares diminuídas do fator transformador de crescimento-alfa (TGF-α) foram observadas em doentes dispépticos, porém não há estudos em populações com SS e RLF.
ObjetivoComparar concentrações salivares do TGF-α; de indivíduos com SS e RLF a de controles saudáveis.
MétodoTrata-se de um estudo prospectivo controlado. Doze pacientes com SS e RLF e 11 indivíduos controles saudáveis tiveram amostras salivares espontâneas e estimuladas coletadas para estabelecer concentração de TGF-α.
ResultadosA concentração salivar de TGF-α; foi estatisticamente maior no grupo estudo para ambas amostras. Este aumento foi confirmado nos sete indivíduos do grupo estudo que não apresentavam esofagite erosiva quando comparados ao grupo controle, porém não houve diferença estatística da concentração de TGF-α; entre pacientes do grupo estudo que apresentavam esofagite erosiva em comparação ao grupo controle.
ConclusãoA concentração salivar de TGF-α; foi estatisticamente maior no grupo de indivíduos com SS e RLF, sem esofagite erosiva.
Saliva performs multiple roles in the digestive system homeostasis.1–6 Examples of its actions include the enzymatic digestion of food; formation of the food bolus; facilitation of mastication, swallowing, and speech; lubrication of mucous membranes; and maintenance of dental health, oral mucosa, and the digestive system. Changes that interfere with the content of saliva may therefore compromise the integrity of this delicate balance and generate consequences in the oral cavity, pharynx, and esophagus.1,2,4,6,7
More than 99% of saliva consists of water, with less than 1% solid elements, mostly proteins and salts.6 The inorganic compounds of saliva are represented mainly by bicarbonate ions, calcium, and phosphate.4,5,8–12 In turn, the organic composition is represented by a series of proteins called growth factors, whose biological action is based on the replication and repair of the epithelium of the digestive system.13 Due to their influence on the protective mechanisms of the digestive system during homeostasis from daily aggressive factors, the most important growth factors in saliva are the family of epidermal growth factors, which comprise epidermal growth factor (EGF) and also transforming growth factor – fraction alpha (TGF-α).14
Salivary TGF-α is a potent mitogenic 50-amino acid polypeptide, whose healing properties are based on its capacity to stimulate DNA synthesis of epithelial cells, with neoangiogenesis and tissue regeneration induction after local injury.15 The literature states that salivary TGF-α exerts its influence on the digestive system by helping to maintain an appropriate pre-epithelial defense barrier, through interaction with other salivary components such as EGF, mucins, and salivary prostaglandins.2,5,6,16
Even though the protective characteristics of TGF-α on the gastric mucosa have been demonstrated,5 there are no data available in the literature regarding the involvement of TGF-α in the physiopathology of supra-esophageal manifestations of gastroesophageal reflux disease (GERD), called laryngopharyngeal reflux (LPR).
As it binds to the same cell receptor as EGF, it can be postulated that salivary TGF-α has a similar influence as salivary EGF on laryngeal protection against the LPR lesions.17 It is known that the biological effects of EGF include ulcer healing, gastric secretion inhibition, and DNA synthesis stimulation, in addition to promoting local mucosal protection against aggressive factors, such as intraluminal gastric acid, biliary acids, pepsin, and trypsin.1,2,6 Studies in patients with LPR observed deficiencies of EGF in the salivary samples compared to samples from healthy individuals, suggesting a protective action of the EGF molecule on the laryngopharyngeal epithelium.9,10,17,18
Another study suggested that there would be a primary salivary EGF deficiency in subjects with LPR that would cause them to be more likely to develop the disease, since after remission of symptoms through the treatment of LPR, these subjects had salivary EGF concentration statistically similar to concentrations found in the active stage of the disease, and significantly lower than that found in normal individuals.19
Aware of the importance of saliva, Rourk16 and Korsten20 observed that states of hyposalivation could increase the risk of gastroesophageal reflux disease (GERD) by concomitant reduction in oroesophageal clearance and mucosal protection. Other authors have reported a greater incidence of caries and periodontitis in patients with hyposalivation.21–23
As the protective protein content is added to the saliva at the level of glandular acini, the search for a clinical experimental model of hyposalivation of acinar origin could contribute to the understanding of the selective role of salivary protein components in the pathogenesis of GERD. With this purpose, it was decided to use Sjögren's syndrome (SS) as a clinical model for the salivary study, as it represents one of the classic forms of known hyposalivation, as well as for having an etiology related to exocrine acinar gland dysfunction (progressive lymphoplasmacytic infiltration and replacement of the excretory parenchyma, particularly of the salivary glands, causing their failure and inducing hyposalivation).6,24
Previous studies have reported a higher prevalence of GERD and its supra-esophageal manifestations (LPR) in populations with SS, suggesting that the association is a frequent one.5,6,20,25–28 For Belafsky and Postma,25 although there is no evidence of primary deficiencies in epithelial resistance, the inflammation of seromucous glands in patients with SS make the laryngeal, pharyngeal, and esophageal mucosa more vulnerable to the harmful effects of refluxate.
Based on the above, the measurement of salivary TGF-α levels in patients with Sjögren's syndrome could help to understand the mechanism of LPR onset in this population, contributing to a better understanding of the physiopathology of this disease and allowing the prevention of possible complications that the associated comorbidities may cause.
This study aimed to analyze the concentration of transforming growth factor – alpha subfraction (TGF-α) in salivary samples of individuals with Sjögren's syndrome and LPR, and compare them with healthy subjects.
MethodsAfter approval by the Ethics Committee on human research of the institution (project number 354/10), a total of 12 subjects with confirmed diagnosis of Sjögren's syndrome and 11 normal controls were included in the study, from August 2010 to February 2013, in a tertiary university hospital.
The diagnosis of SS was made according to the definitions of the European-American consensus of 2002.29 The diagnosis of LPR was made by suggestive laryngopharyngeal symptoms and a compatible video laryngoscopy, confirmed by upper digestive endoscopy (UDE) and/or 24-h dual channel esophageal pH monitoring (pH-metry).14,28–31
All patients who met the inclusion and exclusion criteria were included in the study, after signing the informed consent and receiving information on the study objectives, methodology, and risks.
Inclusion criteria were age >18 years and confirmed clinical, laboratory, and anatomopathological diagnosis of Sjögren's syndrome, as well as the agreement to undergo rigid or flexible video laryngoscopy.
The exclusion criteria were smoking, alcoholism, active infectious or allergic rhinosinusitis, and exposure to abrasive chemical inhalants and chronic lung diseases, as all these factors cause inflammation in the respiratory mucosa, thus mimicking the macroscopic laryngeal and pharyngeal changes found in LPR.30,31 In addition, patients unable to produce sufficient saliva volume for collection and analysis were excluded. Individuals using drugs known to alter the flows of saliva and gastric secretions, such as diuretics, antihistamines, proton-pump inhibitors, prokinetics, antacids, inhaled asthma drugs, neuroleptics, psychotropics, and artificial saliva were also excluded.32
It was also decided to exclude individuals undergoing salivary gland and digestive system surgeries, subjects with pre-neoplastic and neoplastic lesions of the larynx, pharynx, esophagus, stomach, and duodenum (present or previously treated), and patients submitted to prior radiotherapy to prevent other possible methodological biases.17,33 All subjects were evaluated by the same physician, who has experience in performing and interpreting laryngoscopy evaluations.
The control group of 11 healthy female volunteers without Sjögren's syndrome and without GERD or LPR was also studied, taking into account the same abovementioned exclusion criteria.
All individuals were submitted to anamnesis and physical otorhinolaryngological examination, with measurement of weight and height to calculate body mass index (BMI, expressed in kg/m2). Furthermore, patients in the study group were divided into two groups, according to chronic use of drugs potentially damaging to the mucosa of the esophagus and stomach, which may be often used by individuals with SS, such as nonsteroidal anti-inflammatory drugs, methotrexate, chloroquine, and alendronate. This information was used to verify the influence of the abuse of these substances with the occurrence of GERD and LPR in the studied population.
Subsequently, the patients answered a questionnaire about symptoms suggestive of laryngopharyngeal reflux, called the Reflux Symptom Index34 (RSI). A tool that has been validated for the English language, presently undergoing validation process in Brazil, the RSI is a point-based score in which a score >13 indicates the presence of LPR with 95% accuracy.34
Patients then underwent to laryngoscopy with a 10mm and 70° laryngoscope (model precision SN – 29052; Storz – Germany) coupled to a video system (Toshiba, model IK-CU44A – Japan; LG DVD burner, model tri-system – Brazil; and LG monitor, model tri-system CineMaster – Brazil).
A flexible 3.4mm-diameter nasofibrolaryngoscope (EPX 2200 model processor monitor color LCD; Fujinon® – Japan) was used only in cases of overactive gag reflex or incapacity to visualize the laryngopharyngeal segment by telescopy.
All examinations were recorded and stored on DVD media (LG). No topical anesthetic was used during the endoscopic assessment of the larynx.
The presence and severity of signs suggestive of laryngopharyngeal reflux was established by applying a scoring system of endolaryngeal endoscopic inflammatory signs called Reflux Finding Score (RFS),35 or the Scale of Videolaryngoscopy Reflux Findings.36 Similarly to the RSI,34 the RFS35 has been validated for the English language and is undergoing validation in Brazil.36 According to this tool, RFS values >7 suggest the presence of LPR with 95% accuracy.35,36
To maintain the scientific consistency of the project, in addition to the use of the two tools for clinical diagnosis of laryngopharyngeal reflux described above (RSI and RFS), patients underwent additional examination for evidence of GERD and LPR (upper digestive endoscopy – Fujinon® 2200 series, 9.8mm diameter – Japan; and/or manometry/24-h dual-channel esophageal pH monitoring; Alacer Biomedical – São Paulo, Brazil).17,30,34,35,37,38 The presence of erosive esophagitis in patients with RSI and RFS suggestive of reflux confirmed the diagnosis of GERD/LPR.17,30
Patients without endoscopic confirmation of GERD were then subjected to 24-h dual channel esophageal pH-metry preceded by esophageal manometry to locate the lower esophageal sphincter.17,39,40 Proximal pathological reflux was considered as any episode of pH decrease in the proximal sensor to values <4, preceded by an event of the same magnitude in the distal sensor, regardless of its duration.37,41 To determine the distal pathological reflux, pH decrease events in the distal sensor demonstrating levels <4 were also considered, following the criteria described by Demeester et al.42 and according to the parameters for total acid exposure time defined by Jamieson et al.43
Cases with pH-metry that had proximal pathological reflux, isolated or associated with pathological distal reflux, were considered positive for LPR.17,30,40,44
Salivary sample processing and analysisSaliva collection was always performed in the morning after fasting for 8h, in order to comply with the circadian rhythm of salivary production. Patients were instructed not to brush their teeth or use mouthwash in the morning of saliva collection. At the time of collection, the patient was asked to rinse the mouth with running water at will, without swallowing, to reduce excessive cell sloughing present in the oral cavity.
Two consecutive saliva samples were obtained from each participant: the first, of unstimulated saliva (called basal saliva), and the second sample, of stimulated saliva, by chewing a 25cm2 piece of parafilm M® (Pechiney Plastic Packing Chicago, IL, USA), called stimulated saliva.5,16,45
Samples of basal and stimulated saliva were collected by asking the patient to spit at will the entire salivary volume produced during the period of 10min into a universal bottle collector. The patient was instructed not to expectorate or spit nasopharyngeal secretions during the collection period, in order to avoid contaminating the material with secretions that were not salivary.17,18 Moreover, patients were asked to remain silent during the collection period, so as not to interfere with salivary demand.
The processing of saliva was carried out at the same time as collection by centrifugation for 10min (3500rpm – Excelsa II centrifuge – Fanem; Brazil) conditions necessary for sedimentation of cell debris. The supernatant was separated using pipettes to measure the volume and concentration of TGF-α, following the protocol established by Eckley to measure the concentration of salivary factors.5,30
The samples were then stored and maintained at a temperature of −20°C until the time of final analysis of the salivary levels of TGF-α, which was performed by the sandwich ELISA method using the available commercial kit (Quantikine®, R&D Systems Inc. – United States). The method's steps were performed according to the manufacturer's guidelines described in the instruction manual.46
A monoclonal antibody specific for TGF-α had been previously adhered to the wells of a microtiter plate provided by the manufacturer. Provided standard solutions and diluted sample solutions were pipetted in the wells and incubated, so that the TGF-α molecules would adhere to the immobilized antibody in the microtiter plates. After several washings, molecules that had weak bonds with the specific antibodies were removed and a polyclonal antibody specific for TGF-α respectively linked to an enzyme was added to the solution. After further washings, a substrate for the enzyme action was added, so that color development would occur in the solution, in proportion to the amount of TGF-α respectively adhered to the wells at the beginning of processing.
The reaction was then interrupted by adding a specific substrate, and the TGF-α level in each sample was determined based on the optical density of the solutions, recorded by spectrophotometry. This concentration was established as a function of the overall concentration of salivary proteins present in the sample, regardless of the total saliva volume, by comparing optical densities of standard solutions with previously known concentrations and expressed in pg/mL.
The SS patients were subsequently divided into two groups according to the severity of hyposalivation and compared regarding salivary concentrations of TGF-α. Four patients had mild hyposalivation (basal salivary volume between 1 and 3mL and/or stimulated salivary volume between 7 and 15mL), and eight patients had severe hyposalivation (basal salivary volume less than 1mL and/or stimulated salivary volume less than 7mL).25,45,47
These SS patients were further divided into two other groups, according to the presence of erosive esophagitis at the UDE (present in 5 cases and absent in 7). The two subgroups were then compared with each other according to salivary levels of TGF-α.
Statistical analysis was performed using the software Epi Info™ 7 (CDC, Atlanta, USA) and SPSS – release 13 for Windows®. Summary measures for quantitative variables were calculated. For qualitative variables, absolute and relative frequencies were calculated. Parametric and non-parametric statistical methods were used to analyze the variables (Student's t-test and the Mann–Whitney test). The level of statistical significance was set at 5% for all analyses.
ResultsThe study group consisted of 12 individuals diagnosed with SS, with a mean age of 56.25 years±8.6, all females. Five patients were diagnosed with primary SS, and seven with secondary SS, including four cases associated with systemic lupus erythematosus (SLE), two cases associated with rheumatoid arthritis (RA), and one case with RA+SLE association. To characterize the study group, the mean time of diagnosis of Sjögren's syndrome was also obtained at the time of study enrollment. This mean was 5.3±2.2 years. Also for the study group, the mean RSI score was 22.8±6.56 points and the RFS score was 13.3±1.7 points.
The control group consisted of 11 healthy female volunteers with a mean age of 56.1 years±12.7 and without symptoms of GERD/LPR or videolaryngoscopic signs of LPR. The average RSI was 4.2±3.8 points and the average RFS was 3.4±1.9.
The diagnosis of laryngopharyngeal reflux was originally attained in the 12 subjects with Sjögren's syndrome by applying the RFS and RSI scores (all positive for LPR). In five patients, the diagnosis was later confirmed by UDE with erosive esophagitis, and in seven subjects it was confirmed by the presence of pathological reflux in the proximal sensor during the 24-h esophageal pH monitoring with dual sensors. Moreover, eight of these 12 analyzed patients also had the classical form of gastroesophageal reflux disease (five cases with erosive esophagitis at UDE, as indicated above, associated with three cases in which the esophageal pH monitoring showed concomitant distal pathological reflux).
Regarding BMI, the study group had a mean of 26.2±4.2kg/m2, statistically similar to the control group (mean 27.2±4.1kg/m2) (Mann–Whitney test, p=0.806).
Eight of the 12 SS patients assessed had chronic use of potentially harmful drugs to the digestive mucosa. Nevertheless, five of these eight cases did not show alterations during the UDE (Fisher's exact test, p=1).
There was no statistically significant difference between the two groups regarding the age of the studied subjects (Mann–Whitney test, p=0.82), or regarding gender (all were women), which made the groups comparable. In turn, control and study groups were statistically different when the RSI and RFS scores were analyzed (ANOVA p<0.001 for both variables).
The basal salivary volume of the study group (1.6±1.62mL) was significantly lower than that of the control group (2.6±1.44mL) (Mann–Whitney test, p=0.03). In addition, the stimulated salivary volume in the study group (6.7±5.54mL) was also significantly lower than in the control group (9.9±7.29mL) (Mann–Whitney test, p=0.09).
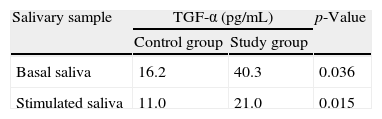
The concentration of TGF-α in the basal saliva was significantly higher in the study group (40.3pg/mL±40.87) compared to the control group (16.2pg/mL±6.59) (Mann–Whitney test, p=0.036). The same was observed in the concentrations of TGF-α in stimulated saliva in the study group (21.0pg/mL±20.83) compared to the control group (11.0pg/mL±1.92) (Mann–Whitney test, p=0.015) (Table 1).
Levels of transforming growth factor alpha (TGF-α) in basal and stimulated saliva samples after chewing parafilm-M in the group of patients with Sjögren's syndrome and confirmed laryngopharyngeal reflux (study group) and the group of healthy volunteers (control group).
| Salivary sample | TGF-α (pg/mL) | p-Value | |
| Control group | Study group | ||
| Basal saliva | 16.2 | 40.3 | 0.036 |
| Stimulated saliva | 11.0 | 21.0 | 0.015 |
TGF-α, transforming growth factor alpha.
When comparing the control group separately with the five cases with erosive esophagitis, it was observed that the mean TGF-α levels in basal and stimulated saliva of the study group with erosive esophagitis (31.2 and 11.8pg/mL, respectively) were statistically similar to those of the control group (16.2 and 11.0pg/mL, respectively) (Mann–Whitney test, p=0.395 for basal saliva and p=0.335 for stimulated saliva).
However, when comparing the control group separately with the seven cases without erosive esophagitis, it was observed that the mean TGF-α levels in basal and stimulated saliva of the study group without erosive esophagitis (46.7 and 23.5pg/mL) were statistically higher than those of the control group (16.2 and 11.0pg/mL) (Mann–Whitney test, p=0.016 for baseline saliva and p=0.005 for stimulated saliva).
In the group with mild hyposalivation, the mean level of TGF-α in saliva at baseline was 13.5±3.34pg/mL, while in the stimulated saliva it was 11.4±1.84pg/mL. When analyzing the group with severe hyposalivation, the values of TGF-α found in the basal and stimulated samples were 53.7±44.8 and 25.7±24.5pg/mL, respectively.
The salivary level of TGF-α in the group with severe hyposalivation was statistically higher in the basal (Mann–Whitney test, p=0.007) and stimulated samples (Mann–Whitney test, p=0.089), when compared to the group with mild hyposalivation.
DiscussionThe high prevalence of GERD and LPR in subjects with SS had already been described by the current research group in an article published in 2012.30 At that time, the presence of LPR was observed in 100% of individuals in a group of 19 adults with SS.30 The sample used in this study was new and included 12 new subjects with SS, all of whom had LPR (eight of whom also had GERD), confirming the high frequency of the association in subpopulations with SS.
In the present study, the presence of GERD was not influenced by BMI, nor by chronic use of medications that are potentially harmful to the mucosa of the esophagus and stomach, suggesting that GERD in patients with SS is apparently not influenced by the same risk factors classically associated with GERD in the general population without xerostomia.
The assessment of TGF-α levels in saliva was motivated by the recognized importance of this growth factor on the digestive system homeostasis, both through its healing and neoangiogenesis-inducing effects, as well as by its inhibitory action against gastric secretion.5,14,48
Moreover, there are no data in the literature analyzing the levels of salivary TGF-α in individuals with LPR, much less comparing this information in a population consisting exclusively of patients with SS.
It is known that the protein content is added to the saliva at the level of the salivary gland acini.6,49 However, it is unclear whether there would be a difference in the rate of salivary excretion of TGF-α according to the type of salivary gland assessed. The exclusive analysis of baseline saliva would mainly evaluate the contribution of submandibular glands to the overall content, while stimulated samples would reflect greater parotid contribution.6 Thus, it was decided to collect basal and stimulated saliva samples to obtain better representation of the overall saliva production.
Regarding the results obtained, in fact, the salivary level of TGF-α in subjects with SS was significantly higher than that in the control group, both in basal and stimulated salivary samples.
Given that the essential role of TGF-α is to stimulate tissue healing, the findings of its increased salivary concentrations in subjects with SS and LPR suggest that this growth factor may participate in the first line of defense in the body's response to aggression resulting from GERD/LPR, also suggesting that its local production plays a significant role in the reconstitution of the laryngeal and pharyngeal mucosa in post-injury repair.
No publications were found that assessed the salivary concentrations of TGF-α and their possible involvement in the genesis of supraesophageal manifestations of GERD.
However, when analyzing the few studies on the participation of this growth factor in the homeostasis of the digestive system, the results observed in this analysis agree with those published by Polk et al., who reported an increase in the production of TGF-α in response to acute gastric aggression with HCl.50 Similar finding was observed by Fujiwara et al., when analyzing mice with chronic reflux esophagitis.15 According to these authors, activation of TGF-α in response to pathological esophageal reflux could facilitate mucosal healing by stimulating epithelial proliferation.15
In the current study, when the group of individuals with SS were subdivided according to the degree of hyposalivation, the objective was to analyze the influence of the most advanced stages of acinar dysfunction on the capacity of TGF-α salivary production and, consequently, on the defense capacity of the laryngopharyngeal and digestive mucosa.
Subjects with severe hyposalivation showed higher concentrations of TGF-α, reinforcing that this growth factor participates in a compensatory defense mechanism. Thus, more advanced stages of salivary dysfunction would qualitatively try to meet the quantitative deficiency of salivary volume produced by the individual.
Considering only patients in the study group with LPR who had erosive esophagitis, no differences were observed in basal and stimulated salivary concentrations of TGF-α when compared to the control group. Furthermore, the subgroup without esophagitis responded to aggression with a significantly higher level of TGF-α in basal and stimulated samples, when compared to the control group.
Assuming that the presence of erosive esophagitis at UDE constitutes a complication of more severe GERD/LPR, the abovementioned facts suggest that the subgroup with esophagitis would have an intrinsic difficulty in increasing the production and/or secretion of salivary TGF-α, necessary to prevent the emergence of this complication. It is assumed, then, that the capacity of the studied subjects with SS to respond to the aggression of GERD/LPR with higher salivary level of TGF-α would have prevented the onset of erosive esophagitis in this study.
Thus, it is possible to hypothesize that the response of an individual with xerostomia to the presence of GERD/LPR would be the increase in salivary TGF-α, as a compensatory effect to prevent disease progression. Cases with an insufficient increase would have complications, such as erosive esophagitis.
In the sample analyzed over a period of three years, there were no SS patients without GERD/LPR. It is postulated that the measurement of salivary TGF-α levels in a subgroup of SS patients without GERD/LPR and their comparison with a group of SS patients with GERD/LPR can be enlightening to disclose the participation of this salivary factor in the biochemical balance of the digestive system. Given the rarity of SS, multicenter studies have been shown to be necessary to increase the representativeness of the findings and prove the scientific hypotheses.
If confirmed in future studies, the results may potentially have significant clinical relevance in the management of patients with SS. Can the measurement of salivary concentrations of TGF-α predict groups of subjects with SS that would present more severe forms of GERD/LPR, and thus, would require more intensive care?
These findings call attention to the fact that the etiology of supraesophageal manifestations of GERD is yet to be fully understood, especially when considered in such a unique group of individuals with SS.
ConclusionIn the present study, the level of TGF-α was statistically higher in the basal and stimulated salivary samples of individuals with Sjögren's syndrome and laryngopharyngeal reflux, when compared to the respective samples from healthy controls.
FundingThis study was funded by Conselho Nacional de Desenvolvimento Cientifico e Tecnológico – CNPq (National Council for Scientific and Technological Development) (grant # 147627/2-10-9).
Conflicts of interestThe authors declare no conflicts of interest.
Please cite this article as: Corvo MA, Eckley CA, Rizzo LV, Sardinha LR, Rodriguez TN, Bussoloti Filho I. Salivary transforming growth factor alpha in patients with Sjögren's syndrome and reflux laryngitis. Braz J Otorhinolaryngol. 2014;80:462–9.